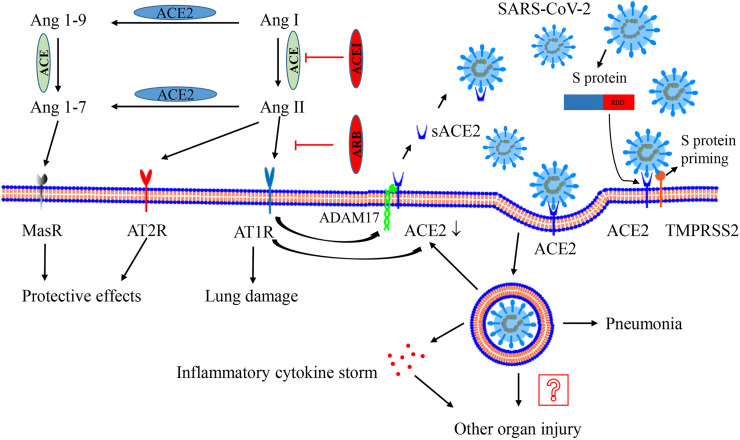
Fig. 1.
Interaction between SARS-CoV-2 and the renin–angiotensin–aldosterone system. ACE metabolizes Ang I to generate Ang II, which mainly binds AT1R to activate the system and result in lung injury. ACE2 could metabolizes Ang II to generate Ang 1–7 and convert Ang I to Ang 1–9. Ang 1–9 is furtherly metabolized to generate Ang 1–7. Ang 1–7 exerts the protective effect on lung injury via binding the receptor MasR. AT2R also has a beneficial effect. The activation of AT1R promotes ADAM17 (as a “sheddase”) to cleave the extracellular domain of surface ACE2, generating sACE2 and reducing surface ACE2 expression. Recombinant sACE2 may be a treatment for SARS-CoV-2. After processing of the S-protein by TMPRSS2, SARS-CoV-2 performs its human-cell entry via binding its S-protein to ACE2 and the RBD of S-protein is responsible for the process. After endocytosis of the viral complex, surface ACE2 is further down-regulated, resulting in unopposed Ang II accumulation. Local activation of the renin–angiotensin–aldosterone system may regulate lung injury responses to viral insults. The virus replicates inside the cell, leading to pneumonia and even inflammatory cytokine storms, which may contribute to other organ injury. Abbreviation: ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; ADAM17, a disintegrin and metalloprotease 17; Ang, angiotensin; AT1R, Ang II type 1 receptor; AT2R, Ang II type 2 receptor; ACEI, ACE inhibitor; ARB, angiotensin receptor blocker; MasR, Mas receptor; TMPRSS2, type II transmembrane serine protease.