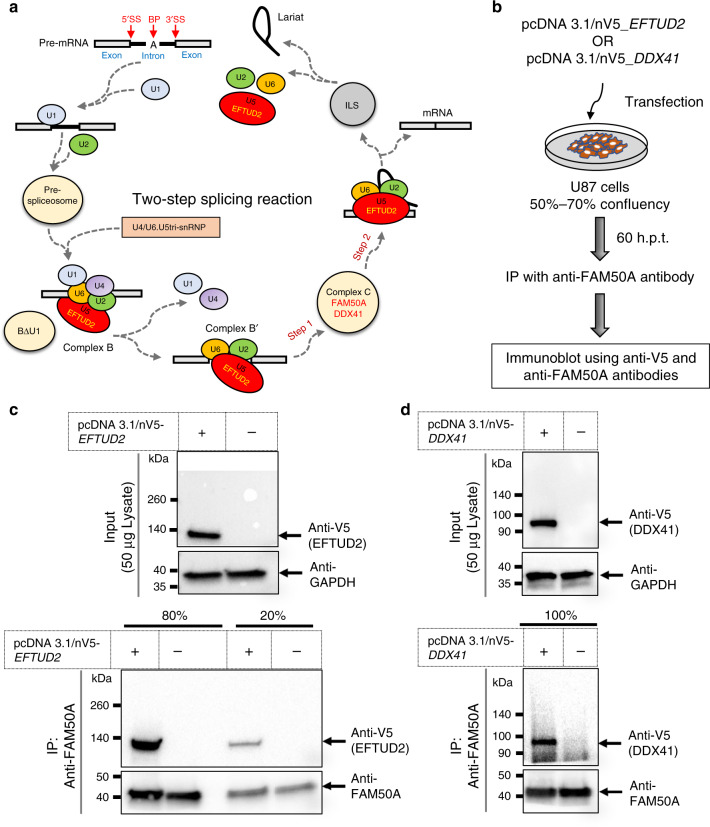
Fig. 6. FAM50A interacts with U5 and C-complex proteins.
a Schematic representation of the two-step splicing reaction (adapted from ref. 70). EFTUD2 (U5); FAM50A and DDX41 (C-complex). b Graphical representation of semi-native co-immunoprecipitation assay. Candidate interactors were overexpressed in U-87 cells, harvested in immunoprecipitation (IP) lysis buffer at 60 h post-transfection and immunoprecipitated with anti-FAM50A antibody. The interaction partners were detected in IP lysate using anti-V5 and anti-FAM50A antibodies, respectively. c, d Western blot of proteins after co-immunoprecipitation. Top: total protein lysate input (50 μg/lane) was migrated on 4–15% polyacrylamide gels to detect EFTUD2 (c) or DDX41 (d) using anti-V5 mouse monoclonal antibody. GAPDH was used as loading control. Bottom: Anti-FAM50A antibody was used to immunoprecipitate native FAM50A protein in total input lysate of 2.3 mg/condition (c) or 3.5 mg/condition (d). The IP lysate was separated in two parts (20% and 80%) and migrated independently on 4–15% polyacrylamide gels. The proteins of interest with predicated band sizes are indicated with black arrows. Plus and minus signs indicate presence and absence of relevant plasmids, respectively. EFTUD2 and DDX41 were detected in independent experiments using protein lysates derived from replicate batches of U-87 cells.