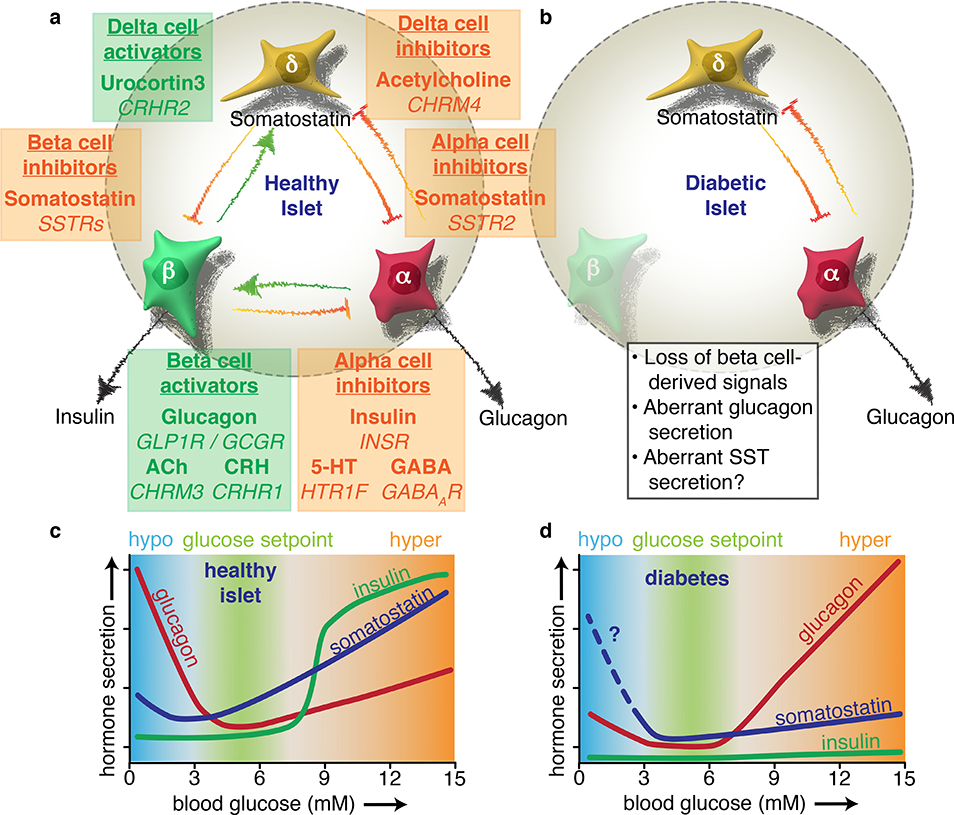
Figure 4. Diabetes disrupts the extensive paracrine signaling network of the islet.
Alpha, beta, and delta cells influence each other’s secretion via intra-islet crosstalk. a) Coloured text boxes (green for activating, orange for inactivating) denote the target cell type of paracrine signaling (underlined), the signal molecule involved (bold), and the target receptor gene (italicised). Each box is placed in between the target cell and the source of the signal. Beta cells initiate a negative feedback loop in high glucose whereby they release urocortin 3 to activate delta cells. The resulting somatostatin (SST) release feeds back to mediate insulin release, providing tonic inhibition that establishes the homeostatic glucose setpoint. Beta cells also experience paracrine activation from alpha cells, which release glucagon, acetylcholine (ACh), and corticotropin-releasing hormone (CRH), which all potentiate GSIS. Beta cell-derived products such as insulin, serotonin (5-HT), and GABA – in addition to urocortin 3-induced somatostatin release – all contribute to silence alpha cells during hyperglycemia. b) The onset of diabetes results in a loss of multiple paracrine signals. Due to autoimmune destruction, type 1 diabetic islets effectively lose all beta cell signals. In type 2 diabetes, urocortin 3 is severely downregulated in beta cells, blunting glucose-stimulated somatostatin secretion. c) The physiological impact of paracrine signaling can be visualised with glucose curves for each islet hormone. The homeostatic glucose set point is maintained by glucagon raising blood glucose during hypoglycaemia and insulin lowering glucose during hyperglycaemia. Somatostatin contributes as a fine-tuning mechanism via paracrine inhibition of both alpha and beta cells. d) The absence of beta cell-derived products in diabetes results in inappropriately high glucagon secretion during high glucose, which exacerbates hyperglycaemia. Glucagon counterregulation at low glucose is also impaired, possibly due to aberrant somatostatin secretion, although this is not yet fully understood. Based in part on Ref17.