Key Points
Question
Does combined chemotherapy and locoregional radiotherapy improve survival of patients with de novo metastatic nasopharyngeal carcinoma compared with chemotherapy alone?
Findings
This phase 3 randomized clinical trial including 126 patients with metastatic nasopharyngeal carcinoma met its primary end point of improved overall survival in favor of chemotherapy plus radiotherapy. Consolidation radiotherapy also improved the secondary end point of progression-free survival, with comparable toxic effects to contemporary intensity-modulated radiation therapy cohorts.
Meaning
The study suggests that local radiotherapy added to chemotherapy improved survival in patients with chemotherapy-sensitive metastatic nasopharyngeal carcinoma.
This randomized clinical trial examines the efficacy and safety of locoregional radiotherapy vs raditherapy alone in patients with de novo metastatic nasopharyngeal carcinoma.
Abstract
Importance
The role of locoregional radiotherapy in patients with de novo metastatic nasopharyngeal carcinoma (mNPC) is unclear.
Objective
To investigate the efficacy and safety of locoregional radiotherapy in de novo mNPC.
Design, Setting, and Participants
Patients with biopsy-proven mNPC, who demonstrated complete or partial response (RECIST v1.1) following 3 cycles of cisplatin and fluorouracil chemotherapy, were enrolled. Eligible patients were randomly assigned (1:1) to receive either chemotherapy plus radiotherapy or chemotherapy alone. Overall, 126 of 173 patients screened were eligible to the study, and randomized to chemotherapy plus radiotherapy (n = 63) or chemotherapy alone (n = 63). Median (IQR) follow-up duration was 26.7 (17.2-33.5) months.
Interventions
The chemotherapy regimens were fluorouracil continuous intravenous infusion at 5 g/m2 over 120 hours and 100 mg/m2 intravenous cisplatin on day 1, administered every 3 weeks for 6 cycles. Patients assigned to the chemotherapy plus radiotherapy group received intensity-modulated radiotherapy (IMRT) after chemotherapy.
Main Outcomes and Measures
The primary end point of the study was overall survival (OS). The secondary end point was progression-free survival (PFS) and safety.
Results
Overall, 126 patients were enrolled (105 men [83.3%] and 21 women [16.7%]; median [IQR] age, 46 [39-52] years). The 24-month OS was 76.4% (95% CI, 64.4%-88.4%) in the chemotherapy plus radiotherapy group, compared with 54.5% (95% CI, 41.0%-68.0%) in the chemotherapy-alone group. The study met its primary end point of improved OS (stratified hazard ratio [HR], 0.42; 95% CI, 0.23-0.77; P = .004) in favor of chemotherapy plus radiotherapy. Progression-free survival was also improved in the chemotherapy plus radiotherapy group compared with the chemotherapy-alone group (stratified HR, 0.36; 95% CI, 0.23-0.57). No significant differences in acute hematological or gastrointestinal toxic effects were observed between the treatment arms. The frequency of acute grade 3 or higher dermatitis, mucositis, and xerostomia was 8.1%, 33.9%, and 6.5%, respectively, in the chemotherapy plus radiotherapy group. The frequency of late severe grade 3 or higher hearing loss and trismus was 5.2% and 3.4%, respectively, in the chemotherapy plus radiotherapy group.
Conclusions and Relevance
In this randomized clinical trial, radiotherapy added to chemotherapy significantly improved OS in chemotherapy-sensitive patients with mNPC.
Trial Registration
ClinicalTrials.gov Identifier: NCT02111460
Introduction
Nasopharyngeal carcinoma (NPC) has a high prevalence in Southeastern Asia, with age-standardized rates of 22.2 to 27.2 per 100 000 male patients.1,2 Incidences of synchronous distant metastasis in endemic NPC range from 6% to 8% at the time of presentation.3,4 In these patients, gemcitabine and cisplatin (GP) doublet chemotherapy is the standard of care (SOC) as first-line treatment.5 Response rates and overall survival (OS) were superior with GP compared with fluorouracil and cisplatin (PF). Median OS of de novo metastatic NPC (mNPC) is approximately 29.1 months with GP.
Local therapy has been used for metastatic disease with the intent of reducing primary tumor burden, relieving symptoms, or propagation of metastases.6 Some studies have demonstrated that intensive local therapy could prolong OS in treatment-naive metastatic cancers.6,7,8,9 This concept is supported by 2 randomized clinical trials reporting the OS benefit of high-dose radiotherapy to the metastatic lesions (COMET)10 or the primary tumor (STAMPEDE).11 Of note, patients in the COMET trial were enrolled only if they demonstrated complete or partial response to systemic therapy, whereas in the STAMPEDE trial, the benefit of local radiotherapy was solely observed in men with low-volume metastatic prostate cancer. Nonetheless, these studies did not consist of patients with NPC.
Several retrospective series have indicated an improvement of 17.0% to 25.0% in 2-year OS with combination radiotherapy and systemic therapy than systemic therapy alone in patients with mNPC.12,13,14,15 However, these studies were retrospective, and varied in terms of clinical heterogeneity of patients, treatment regime, and radiotherapy coverage (primary tumor only or primary tumor and neck) and doses. We therefore conducted a multicenter, randomized phase 3 clinical trial investigating the efficacy of locoregional radiotherapy to the primary tumor and nodal regions in patients with mNPC who demonstrated an initial complete or partial response to palliative PF chemotherapy. Treatment with PF was assigned as the control arm in this trial because this study preceded the data by Li and colleagues.5
Methods
Study Design
The trial protocol is available in Supplement 1. This is a 3-center, randomized, open-label, phase 3 trial (eTable 3 in Supplement 2). The study protocol was approved by the ethics committee of the Sun Yat-sen University Cancer Center (SYSUCC). The trial was performed in accordance with the Declaration of Helsinki, and the results are reported according to the CONSORT statement. All participants provided written informed consent.
Patient Selection
Patients were eligible if they had histopathologically confirmed mNPC (stage IVc by the International Union Against Cancer/American Joint Committee on Cancer staging systems for NPC 7th edition). Inclusion criteria included (1) complete response (CR) or partial response (PR) by an imaging study after 3 cycles of PF; (2) no prior anticancer treatment; (3) Karnofsky performance status (KPS) score of 70 or higher; (4) ages 18 to 65 years; (5) adequate organ function. Exclusion criteria included (1) prior definitive radiotherapy or chemoradiotherapy for NPC; (2) life-threatening medical conditions; (3) patients who were pregnant or breastfeeding; (4) medical history of other malignant diseases within the past 5 years, except for basal cell carcinoma, cervical carcinoma in situ, and superficial bladder tumors.
All patients were screened and recruited at the point of diagnosis. Pretreatment evaluation included a complete medical history and physical examination; hematologic and biochemical analyses; nasopharyngoscopic findings; and magnetic resonance imaging (MRI) or contrast-enhanced computed tomographic (CT) imaging if patients had contraindications to MRI of the head and neck. 18F-fluorodeoxyglucose positron emission tomographic imaging (18F-FDG-PET-CT) was mandatory for distant metastasis staging.
Randomization and Masking
Patients who achieved a CR/PR after 3 cycles of PF were enrolled. Randomization was performed at the Clinical Trials Centre of SYSUCC by a computer-generated random number code. Details of the group allocations were contained in sequentially numbered, opaque, sealed envelopes prepared by a data management team that was blinded to the patient allocation process, and had no clinical involvement with the trial. Patients were randomized 1:1 to either PF alone (chemotherapy-alone group) or PF combined with locoregional radiotherapy (chemotherapy plus radiotherapy group) with a block size of 6 (known only to the data management team). Stratification factors included treatment centers (SYSUCC, Guangdong Provincial People’s Hospital, and The First Affiliated Hospital, Sun Yat-sen University), chemotherapy response (CR vs PR), and number of distinct metastatic lesions (1-2 vs ≥3) determined at the time of randomization.
Procedures
The PF regimen was 5 g/m2 of fluorouracil via a continuous intravenous infusion over 120 hours and an intravenous administration of 100 mg/m2 of cisplatin on day 1. Patients in both groups received their allocated treatment once every 3 weeks for a maximum of 6 cycles, until disease progression, death, dose-limiting toxic effects, or at patient’s request to stop. Off-protocol anticancer drugs were not allowed before the occurrence of protocol-defined disease progression. Details of the chemotherapy dose modifications and supportive measures are provided in the eAppendix in Supplement 2.
Patients assigned to the chemotherapy plus radiotherapy group received intensity-modulated radiotherapy (IMRT) after chemotherapy. Time to commencement of radiotherapy from the end of last chemotherapy cycle was set at 21 days. The IMRT target volumes were delineated using prechemotherapy imaging data according to a previously described institutional treatment protocol.16,17 Briefly, the primary NPC tumor (including retropharyngeal nodes; GTVnx) and gross cervical lymph nodes (GTVnd) were delineated. The high-risk clinical tumor volume (CTV1) was then defined by the prechemotherapy GTVnx with a 0.5- to 1.0-cm margin (0.2-0.3 cm posterior margin). The low-risk clinical target volume (CTV2) was defined as CTV1 plus a 0.5- to 1.0-cm margin (0.2- to 0.3-cm posterior margin) to encompass the at-risk regions at the base of skull and parapharyngeal regions, and the involved and at-risk cervical nodal levels (levels II to Vb, and the supraclavicular fossa). Planning target volume margins (PTV1 and PTV2) were 0.5 cm circumferentially and 0.3 cm posteriorly. Prescribed doses were 70 Gy to GTVnx, 60 to 66 Gy to GTVnd, 56 to 66 Gy to PTV1, and 50 to 60 Gy to PTV2 in 33 fractions, 5 times per week. Complete details on the radiotherapy planning are provided in the eAppendix in Supplement 2.
Tumor response after 3 cycles of PF was based on the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (version 1.1), and assessed by nasopharyngoscopy and MRI for the primary site, and 18F-FDG-PET-CT or CT or MRI for the distant lesions.
Patients were followed up every 2 to 3 months until death to evaluate the efficacy and safety of the treatment. We recorded the survival status, and subsequent lines of therapies. Local treatment for primary or metastatic lesions was allowed in the chemotherapy only arm for palliation of symptoms. Adverse events were scored according to the Common Terminology Criteria for Adverse Events (CTCAE; version 3.0) and Late Radiation Morbidity Scoring Scheme of the Radiation Therapy Oncology Group at each follow-up visit.
Study End Points
The primary end point of the study was OS, which was defined as the time interval from randomization to death due to any cause. Imaging results to assess the secondary end points of PFS and objective response rate (ORR) were centrally reviewed. Progression-free survival was defined as time from randomization to locoregional or distant metastasis relapse or death from any cause, whichever occurred first. Objective response rate referred to the proportion of patients who had a confirmed objective response (defined as CR or PR from the first evaluation after 3 cycles of PF). Patients who were alive and without a recorded event were censored at the date of last follow-up.
Statistical Plan
The primary aim of this study was to test if systemic chemotherapy plus radiotherapy improved OS over systemic chemotherapy alone. Based on previous reports,14,15,18 we assumed that 2-year OS rate was 51.0% for patients treated with systemic chemotherapy alone, and 70.0% for patients treated with systemic chemotherapy plus radiotherapy, which corresponded to a target hazard ratio (HR) of 0.53. The log-rank test was used to calculate the sample size. The expected length of accrual period was 3 years, and the expected maximum length of follow-up was 5 years. The 2-sided type I error was 0.05 (α = 0.05), and the power was 0.90 (1-β). After accounting for a 10% dropout rate, we estimated that a total of 204 participants were needed, (102 participants in each group), with 104 events observed for the primary analysis of OS.
Categorical variables were compared using the χ2 test or Fisher exact test. Continuous variables were compared using the Mann-Whitney U test. All patients randomly assigned to a group (the intention-to-treat population) were included in the primary efficacy assessment. For all patients, the median follow-up time was calculated using the reverse Kaplan-Meier method. Kaplan-Meier curves were used to present time-to-event data, and compared by means of a log-rank test. The stratified Cox proportional hazards model, with treatment response and number of metastatic lesions as covariates, was used to calculate the stratified HRs and 95% CIs , and the proportional-hazards assumption was tested with Schoenfeld residuals.19
We further performed an interaction analysis to explore whether the effect of the experimental treatment varied in the subgroups defined according to sites and number of metastatic lesions, and treatment response. The interaction analysis was conducted by means of a test of treatment-by-covariate interaction on the basis of the Cox proportional-hazards model.20
The O’Brien-Fleming and Pocock methods for 1 interim analysis were used to rerun the post hoc interim analysis to test the robustness of the unplanned interim analysis by potentially inflating the type I error. All of the analyses were performed using Stata statistical software (version 14.2, StataCorp). Two-tailed P < .05 was considered statistically significant. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number RDDA2020001470.
Early Closure to New Patient Enrollment
In August 2018, 126 eligible patients of the initially planned 204 patients had been enrolled. The independent data monitoring committee (IDMC), who were blinded to the assigned treatment groups, recommended temporarily suspending randomization, acquiring additional follow-up data owing to an imbalance in deaths between the 2 groups and notifying the SYSUCC ethics committee. On February 2019, the IDMC confirmed the previously identified imbalance with additional follow-up data. The IDMC and the ethics committee (which was also blinded) of SYSUCC (eTables 4 and 5 in Supplement 2) therefore recommended premature closure of the trial.
Results
Patients and Treatment
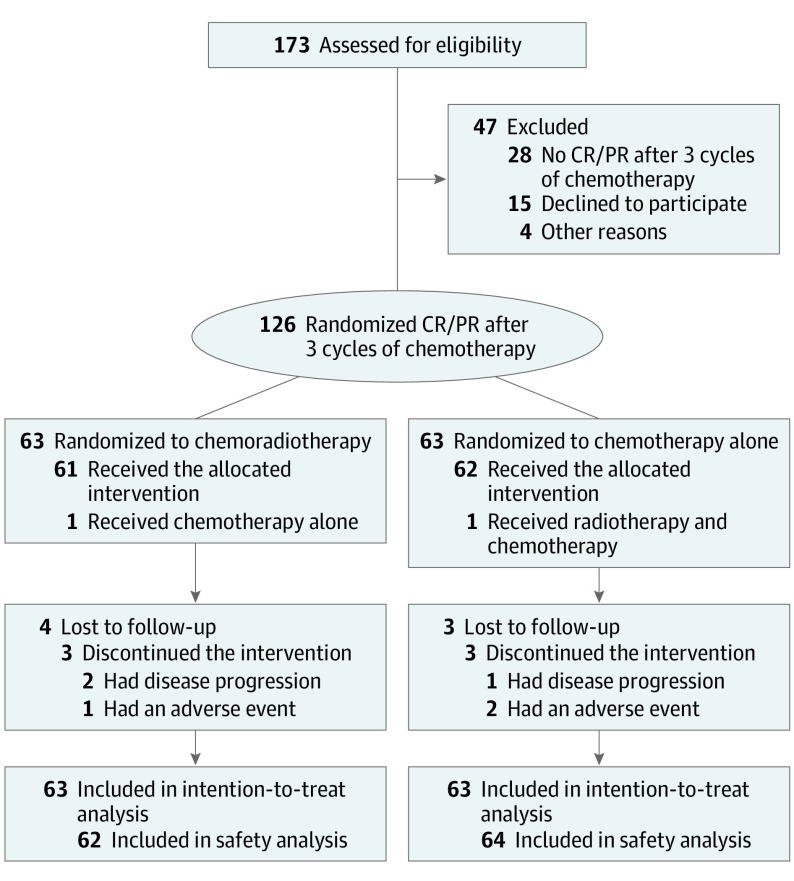
Between April 2014 and August 2018, 173 patients diagnosed with mNPC from 3 centers were screened for eligibility. Forty-seven patients were excluded because they either did not achieve a CR or PR, as evaluated by imaging, after 3 cycles of PF (n = 28), or declined to participate (n = 15), or excluded for other reasons (n = 4). The remaining 126 eligible patients were randomly assigned to receive chemotherapy plus radiotherapy (n = 63) or chemotherapy alone (n = 63). One patient assigned to the chemotherapy-alone group chose to receive chemotherapy plus radiotherapy. Two patients assigned to the chemotherapy plus radiotherapy group chose to receive chemotherapy alone. These patients were included in the efficacy analysis according to their assigned groups, and included in the safety analysis according to the regimens they actually received (Figure 1).
Figure 1. Flow Chart of Trial Participants.
CR indicates complete response; PR, partial response.
Baseline demographics and disease characteristics were balanced between the treatment groups (Table 1). Median age was 46.0 (IQR 37.0-52.0) years in the chemotherapy plus radiotherapy group and 47.0 (IQR 39.0-52.0) years in the chemotherapy-alone group. In the chemotherapy plus radiotherapy group, 62 of the 63 patients (98.4%) completed all 6 cycles of PF; 1 patient received 4 cycles because of disease progression in the chemotherapy plus radiotherapy group. In the chemotherapy-alone group, 60 of the 63 patients (95.2%) completed all 6 cycles of PF chemotherapy, and 3 patients received 5 cycles (1 patient developed disease progression, and 2 discontinued owing to adverse events). Median cumulative dose intensity for cisplatin was 560 (IQR, 520-600) mg/m2 in the chemotherapy plus radiotherapy group and 540 (IQR, 500-600) mg/m2 in the chemotherapy-alone group. Median cumulative dose intensity for fluorouracil was 5500 (IQR, 5000-6000) mg/m2 and 5600 (IQR, 5000-6000) mg/m2, respectively. Fifty-nine of the 61 patients (96.7%) completed protocol-defined IMRT (2 patients discontinued because of disease progression and toxic effects) (eTable 6, eFigure 1 in Supplement 2).
Table 1. Baseline Demographics and Disease Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Chemotherapy plus radiotherapy (n=63) | Chemotherapy alone (n=63) | |
| Sex | ||
| Female | 10 (15.9) | 11 (17.5) |
| Male | 53 (84.1) | 52 (82.5) |
| Age, median (IQR), y | 46.0 (37.0-52.0) | 47.0 (39.0-52.0) |
| Karnofsky performance status score | ||
| 90-100 | 58 (92.1) | 57 (90.5) |
| 70-80 | 5 (7.9) | 6 (9.5) |
| Smoking status | ||
| Smokers | 22 (34.9) | 20 (31.7) |
| Nonsmokers | 41 (65.1) | 43 (68.3) |
| Histologic findings | ||
| Nonkeratinizing undifferentiated (type III) | 60 (95.2) | 61 (96.8) |
| Nonkeratinizing differentiated (type II) | 2 (3.2) | 1 (1.6) |
| Keratinizing (type I) | 1 (1.6) | 1 (1.6) |
| T classification | ||
| T1-T2 | 7 (11.1) | 8 (12.7) |
| T3-T4 | 56 (88.9) | 55 (87.3) |
| N classification | ||
| N0-N1 | 14 (22.2) | 11 (17.5) |
| N2-N3 | 49 (77.8) | 52 (82.5) |
| Bone metastases | ||
| No | 19 (30.2) | 16 (25.4) |
| Yes | 44 (69.8) | 47 (74.6) |
| Liver metastases | ||
| No | 45 (71.4) | 44 (69.8) |
| Yes | 18 (28.6) | 19 (30.2) |
| Lung metastases | ||
| No | 45 (71.4) | 46 (73.0) |
| Yes | 18 (28.6) | 17 (27.0) |
| Treatment responsea | ||
| Complete | 3 (4.8) | 4 (6.3) |
| Partial | 60 (90.4) | 59 (93.7) |
| Metastatic lesionsa | ||
| 1-2 | 19 (30.1) | 20 (31.7) |
| ≥3 | 44 (69.8) | 43 (68.3) |
Abbreviation: IQR, interquartile range.
Treatment response and number of metastatic lesions were evaluated after 3 cycles of chemotherapy (at randomization), whereas remaining other clinical characteristics were evaluated before chemotherapy.
Efficacy
The data cut-off date for the analysis was August 2019. A total of 103 of 126 patients (81.7%) had follow-up records of at least 24 months including 51 recorded deaths (17 in chemotherapy plus radiotherapy group, 34 in chemotherapy-alone group; all patients died from NPC), 7 patients recorded lost to follow-up (4 in chemotherapy plus radiotherapy group and 3 in chemotherapy-alone group) and 45 patients remained alive. The median follow-up time was 26.7 (IQR 17.2-33.5) months. The 24-month OS was 76.4% (95% CI, 64.4%-88.4%) in chemotherapy plus radiotherapy group, compared to 54.5% (95% CI, 41.0%-68.0%) in chemotherapy-alone group (stratified hazard ratio for death, 0.42; 95% CI; 0.23-0.77, P = .004, Figure 2). Ninety-three patients had documented disease progression (37 in the chemotherapy plus radiotherapy group and 56 in the chemotherapy-alone group). Patients in the chemotherapy plus radiotherapy group had a superior PFS compared with those in the chemotherapy group (stratified HR, 0.36; 95% CI, 0.23-0.57; P < .001). Patterns of relapse are summarized in the eTable 7 in Supplement 2.
Figure 2. Overall Survival and Progression-free Survival in the Intention-to-Treat Population.
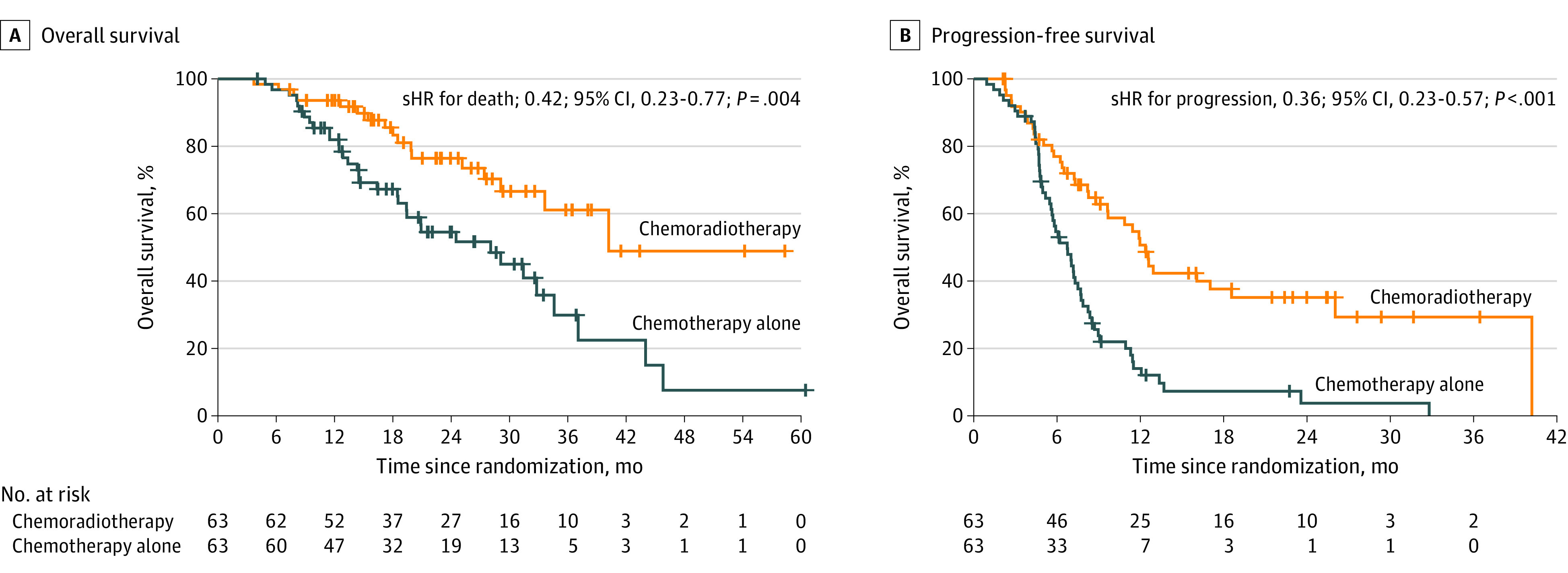
sHR indicates stratified hazard ratio.
The ORR was comparable between the treatment groups at the end of 6 cycles of chemotherapy (80.9% vs 82.5%). On completion of radiotherapy, the overall ORR was 10 CR (16.4%), 36 PR (59.0%), 5 stable disease (8.2%), 8 progressive disease (13.1%); and 2 cases were nonevaluable (Table 2).
Table 2. Efficacy of Study Treatment.
| End point | No. (%) | HR (95% CI)a | |
|---|---|---|---|
| Chemotherapy plus radiotherapy (n=63) | Chemotherapy alone (n=63) | ||
| Overall survival | |||
| Deaths | 17 (27.0) | 34 (54.0) | |
| OS rate, % (95% CI) | |||
| 6 mo | 98.4 (95.2-100.0) | 96.8 (92.5-100.0) | |
| 12 mo | 93.6 (87.5-99.7) | 81.9 (72.3-91.5) | |
| 24 mo | 76.4 (64.4-88.4) | 54.5 (41.0-68.0) | 0.42 (0.23-0.77) |
| Progression-free survival | |||
| Failures | 37 (58.7) | 56 (88.9) | |
| PFS, Median (95% CI), mo | 12.4 (10.5-14.2) | 6.7 (5.4-8.0) | |
| PFS rate, % (95% CI) | |||
| 6 mo | 76.9 (66.3-87.5) | 54.6 (42.1-67.1) | |
| 12 mo | 50.6 (37.3-63.9) | 13.9 (4.7-23.1) | |
| 24 mo | 35.0 (21.7-48.3) | 3.6 (0-9.7) | 0.36 (0.23-0.57) |
| Response to treatment (at the end of chemotherapy) | |||
| Complete response | 5 (7.9) | 4 (6.3) | NA |
| Partial response | 46 (73.0) | 48 (76.2) | NA |
| Stable disease | 5 (7.9) | 2 (3.2) | NA |
| Progressive disease | 7 (11.1) | 9 (14.3) | NA |
| Overall response | 51 (80.9) | 52 (82.5) | NA |
| Disease control | 56 (88.9) | 54 (85.7) | NA |
| Response to treatment (at the end of radiotherapy)b | |||
| Complete response | 10 (16.4) | NA | NA |
| Partial response | 36 (59.0) | NA | NA |
| Stable disease | 5 (8.2) | NA | NA |
| Progressive disease | 8 (13.1) | NA | NA |
| Not assessable | 2 (3.3) | NA | NA |
| Overall response | 46 (75.4) | NA | NA |
| Disease control | 51 (83.6) | NA | NA |
Abbreviations: HR, hazard ratio; NA, not applicable; OS, overall survival; PFS, progression-free survival.
Hazard ratio, which was calculated using the stratified Cox proportional hazards model.
Because there were 2 patients who did not receive radiotherapy in the chemotherapy plus radiotherapy group, a total of 61 patients were included in the analysis of treatment response at the end of radiotherapy. The response at the end of radiotherapy indicates the overall response.
Thirty-six (57.1%) of 63 patients in the chemotherapy plus radiotherapy group and 41 (65.1%) of 63 patients in the chemotherapy-alone group received second-line chemotherapy at documented progression, although median time to subsequent therapy was longer in the experimental group (11.8 months vs 7.7 months) (eTable 8 in Supplement 2). Four patients in the control group received palliative local-regional radiotherapy for symptom control.
Finally, we evaluated OS results with commonly used α spending functions. For 51 of 104 (49.0%) of the required events, the α values used for the O’Brien-Fleming and Pocock methods were 0.005 and 0.031, respectively. Under both αvalues, our results for the primary end point remained significant.
Adverse Events
Overall, 62 patients in the chemotherapy plus radiotherapy group and 64 patients in the chemotherapy-alone group were included in the safety analysis (Table 3). There were no significant differences in hematologic toxic effects between the treatment groups. Overall, grade 3 to 4 neutropenia was the most common toxic effects, which occurred in 35 (56.5%) patients in the chemotherapy plus radiotherapy group, and 35 (54.7%) patients in the chemotherapy-alone group. No significant differences between both groups were observed for hepatotoxic, nephrotoxic, and gastrointestinal toxic effects. Specific to the radiotherapy adverse effects, we observed 5 (8.1%) acute grade 3 or higher dermatitis, 21 (33.9%) grade 3 or higher mucositis, and 4 (6.5%) grade 3 or higher xerostomia. Regarding late adverse events, we recorded 3 (5.2%) grade 3 or higher hearing loss, and 2 (3.4%) grade 3 or higher trismus.
Table 3. Adverse Events According to Trial Group and Grade.
| Event | Patients with event, No. (%) | |||||
|---|---|---|---|---|---|---|
| Chemotherapy plus radiotherapy (n = 62) | Chemotherapy alone (n = 64) | |||||
| Grade 0 or 1 | Grade 2 | Grade 3 or 4 | Grade 0 or 1 | Grade 2 | Grade 3 or 4 | |
| Acute hematologic toxicity | ||||||
| Anemia | 24 (38.7) | 20 (32.3) | 18 (29.1) | 28 (43.8) | 20 (31.3) | 16 (25.0) |
| Thrombocytopenia | 45 (72.6) | 8 (12.9) | 9 (14.6) | 48 (75.0) | 7 (10.9) | 9 (14.1) |
| Neutropenia | 15 (24.2) | 12 (19.4) | 35 (56.5) | 19 (29.7) | 10 (15.6) | 35 (54.7) |
| Leukopenia | 20 (32.3) | 30 (48.4) | 12 (19.3) | 26 (40.6) | 25 (39.1) | 13 (20.3) |
| Acute gastrointestinal reactions | ||||||
| Nausea | 36 (58.1) | 19 (30.6) | 7 (11.3) | 38 (59.4) | 18 (28.1) | 8 (12.5) |
| Vomiting | 41 (66.1) | 16 (25.8) | 5 (8.1) | 43 (67.2) | 17 (26.6) | 4 (6.3) |
| Diarrhea | 59 (95.2) | 2 (3.2) | 1 (1.6) | 62 (96.9) | 2 (3.1) | 0 |
| Acute hepatotoxic effects | 52 (83.9) | 6 (9.7) | 4 (6.5) | 54 (84.4) | 7 (10.9) | 3 (4.7) |
| Acute nephrotoxic effects | 54 (87.1) | 8 (12.9) | 0 | 59 (92.2) | 5 (7.8) | 0 |
| Acute toxic effects specific to radiotherapy | ||||||
| Skin reaction | 47 (75.8) | 10 (16.1) | 5 (8.1) | NA | NA | NA |
| Mucositis | 29 (46.8) | 12 (19.4) | 21 (33.9) | NA | NA | NA |
| Weight loss | 49 (79.0) | 13 (21.0) | 0 | NA | NA | NA |
| Dry mouth | 28 (45.2) | 30 (48.4) | 4 (6.5) | NA | NA | NA |
| Late toxic effects specific to radiotherapya | ||||||
| Cranial neuropathy | 55 (95.0) | 3 (5.2) | 0 | NA | NA | NA |
| Eye damage | 58 (100) | 0 | 0 | NA | NA | NA |
| Dry mouth | 48 (82.8) | 10 (17.2) | 0 | NA | NA | NA |
| Neck tissue damage | 52 (90.0) | 6 (10.3) | 0 | NA | NA | NA |
| Trismus | 53 (91.4) | 3 (5.2) | 2 (3.4) | NA | NA | NA |
| Deafness | 49 (84.5) | 6 (10.3) | 3 (5.2) | NA | NA | NA |
Abbreviation: NA, not applicable.
Only 58 patients were assessed by late toxic effects specific to radiotherapy.
Discussion
To our knowledge, this is the first randomized phase 3 clinical trial evaluating the efficacy of definitive locoregional IMRT when added to the backbone of palliative chemotherapy in patients with de novo mNPC. We demonstrated that high-dose IMRT to the primary and nodal regions resulted in a significant OS advantage (stratified HR, 0.42) in a carefully selected subgroup of patients with mNPC, who had demonstrated initial sensitivity to PF chemotherapy. This result corresponds to an improved PFS in the combination treatment group compared with the chemotherapy-alone group (stratified HR, 0.36). Treatment compliance was high in this trial. Median cumulative dose intensities for cisplatin were 560 mg/m2 and 540 mg/m2 in the experimental and control arms, respectively. The high tolerance to cisplatin could be owing to strict hydration and the use of furosemide and mannitol to prevent cisplatin-induced nephrotoxic effects. Protocol-defined chemotherapy dose adjustments were also strictly adhered to. These measures would have accounted for the low rates of severe gastrointestinal and renal toxic effects in this trial. Our rates of 33.9% for grade 3 to 4 mucositis and 24.2% for grade 2 or higher dermatitis secondary to radiotherapy, were comparable with contemporary studies.21,22 Incidence of deafness was also low, but this may be owing to the short duration of follow-up.23,24 Collectively, these results support a new SOC for chemotherapy-sensitive de novo mNPC.
Prior to this clinical trial, several retrospective series had reported the outcomes of consolidation radiotherapy in patients with mNPC.12,13,14,15 Nonetheless, these retrospective studies were biased by patient selection, treatment variation in chemotherapy intensities, sequencing strategies, and radiotherapy doses. The present study was thus designed to investigate a treatment strategy involving sequential chemotherapy and radiotherapy such that full dosing of both modalities could be achieved.
What is the mechanism underpinning the effect of treating the primary tumor on the overall disease trajectory of metastatic disease? Importantly, apart from an expected reduction of locoregional relapses, locoregional radiotherapy also resulted in fewer distant metastatic recurrences (54.0% vs 68.3%). These observations suggest a possible mechanism that targeting the index tumor lesion could delay the seeding of subsequent tumor clones at distant sites. It has been proposed that soluble growth factors produced by the primary tumor do promote clustering of hematopoietic progenitor cells and macrophages, thereby creating an environment conducive for the dissemination of malignant clones.25,26 Future work is needed to understand the mechanisms contributing to this observed synergy in the clinic.
Limitations
Several points of this trial require highlighting. Foremost, the role of locoregional treatment among the chemotherapy non-responders remains undefined. Alluding to this, of the 7 patients who received high-dose IMRT despite disease progression between the third and sixth cycle of PF, 5 showed metastatic progression within 3 months post-IMRT. Locoregional treatment is thus of limited benefit in this subgroup of patients. Second, although local treatment was allowed for patients in the control arm for symptom palliation, its impact on the primary and secondary end points of the trial is likely minimal because nonradical radiation doses were prescribed, and only 4 patients received this treatment. Third, we acknowledged that the study findings were based on an unplanned interim analysis due to early trial closure. However, this was endorsed by the independent IDMC and ethics committee, who were blinded to the treatment arms. Finally, PF was used instead of GP as the SOC because our study was planned prior to release of the trial results by Li and colleagues.5 However, it would be reasonable to assume the same survival benefit of high-dose locoregional IMRT in combination with GP.5
Conclusions
These findings suggest that chemotherapy plus high-dose locoregional radiotherapy improves survival in chemotherapy-sensitive patients with de novo mNPC.
Trial Protocol
eAppendix 1. Chemotherapy dose modification
eAppendix 2. Intensity-modulated radiation therapy (IMRT) planning protocol in this trial
eFigure 1. Median relative dose intensity of cisplatin and 5-fluorouracil in each cycle
eFigure 2. Forest plots of treatment effects on Overall Survival within subgroups
eTable 1. Chemotherapy and local treatment in primary metastasized malignancies
eTable 2. Chemotherapy plus local radiotherapy in metastatic NPC
Table 3. Distribution of patients by site of enrollment
Table 4. Members of the Independent Data Monitoring Committee (IDMC)
Table 5. Members of the ethics committee of SYSUCC
Table 6. Treatment exposure in the intention-to-treat population
Table 7. Disease recurrence distribution in the two treatment groups
Table 8. Summary of subsequent therapies
Data sharing statement
References
- 1.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30(2):114-119. doi: 10.5732/cjc.010.10377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012-1024. doi: 10.1016/S0140-6736(15)00055-0 [DOI] [PubMed] [Google Scholar]
- 3.Lee AW, Poon YF, Foo W, et al. . Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23(2):261-270. doi: 10.1016/0360-3016(92)90740-9 [DOI] [PubMed] [Google Scholar]
- 4.GLOBOCAN cancer statistics. https://globocan.iarc.fr/Pages/fact_sheets population.aspx. Accessed January 2, 2017.
- 5.Zhang L, Huang Y, Hong S, et al. . Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883-1892. doi: 10.1016/S0140-6736(16)31388-5 [DOI] [PubMed] [Google Scholar]
- 6.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R; European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group . Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358(9286):966-970. doi: 10.1016/S0140-6736(01)06103-7 [DOI] [PubMed] [Google Scholar]
- 7.Flanigan RC, Salmon SE, Blumenstein BA, et al. . Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655-1659. doi: 10.1056/NEJMoa003013 [DOI] [PubMed] [Google Scholar]
- 8.Gomez DR, Blumenschein GR Jr, Lee JJ, et al. . Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672-1682. doi: 10.1016/S1470-2045(16)30532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seisen T, Sun M, Leow JJ, et al. . Efficacy of high-intensity local treatment for metastatic urothelial carcinoma of the bladder: a propensity score-weighted analysis from the National Cancer Data Base. J Clin Oncol. 2016;34(29):3529-3536. doi: 10.1200/JCO.2016.66.7352 [DOI] [PubMed] [Google Scholar]
- 10.Palma DA, Olson R, Harrow S, et al. . Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-2058. doi: 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 11.Parker CC, James ND, Brawley CD, et al. ; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators . Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366. doi: 10.1016/S0140-6736(18)32486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rusthoven CG, Lanning RM, Jones BL, et al. . Metastatic nasopharyngeal carcinoma: patterns of care and survival for patients receiving chemotherapy with and without local radiotherapy. Radiother Oncol. 2017;124(1):139-146. doi: 10.1016/j.radonc.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 13.Zou X, You R, Liu H, et al. . Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur J Cancer. 2017;77:117-126. doi: 10.1016/j.ejca.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 14.Lin S, Tham IW, Pan J, Han L, Chen Q, Lu JJ. Combined high-dose radiation therapy and systemic chemotherapy improves survival in patients with newly diagnosed metastatic nasopharyngeal cancer. Am J Clin Oncol. 2012;35(5):474-479. doi: 10.1097/COC.0b013e31821a9452 [DOI] [PubMed] [Google Scholar]
- 15.Chen MY, Jiang R, Guo L, et al. . Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer. 2013;32(11):604-613. doi: 10.5732/cjc.013.10148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai SZ, Li WF, Chen L, et al. . How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661-668. doi: 10.1016/j.ijrobp.2010.03.024 [DOI] [PubMed] [Google Scholar]
- 17.Xiao WW, Huang SM, Han F, et al. . Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117(9):1874-1883. doi: 10.1002/cncr.25754 [DOI] [PubMed] [Google Scholar]
- 18.Setton J, Wolden S, Caria N, Lee N. Definitive treatment of metastatic nasopharyngeal carcinoma: Report of 5 cases with review of literature. Head Neck. 2012;34(5):753-757. doi: 10.1002/hed.21608 [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239-241. doi: 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 20.Fisher DJ, Copas AJ, Tierney JF, Parmar MK. A critical review of methods for the assessment of patient-level interactions in individual participant data meta-analysis of randomized trials, and guidance for practitioners. J Clin Epidemiol. 2011;64(9):949-967. doi: 10.1016/j.jclinepi.2010.11.016 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Chen L, Hu GQ, et al. . Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med. 2019;381(12):1124-1135. doi: 10.1056/NEJMoa1905287 [DOI] [PubMed] [Google Scholar]
- 22.Lee AW, Ngan RK, Tung SY, et al. . Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 2015;121(8):1328-1338. doi: 10.1002/cncr.29208 [DOI] [PubMed] [Google Scholar]
- 23.Young YH, Lou PJ. Post-irradiation sudden deafness. J Laryngol Otol. 1999;113(9):815-817. doi: 10.1017/S0022215100145281 [DOI] [PubMed] [Google Scholar]
- 24.Cheng JC, Chao KS, Low D. Comparison of intensity modulated radiation therapy (IMRT) treatment techniques for nasopharyngeal carcinoma. Int J Cancer. 2001;96(2):126-131. doi: 10.1002/ijc.1004 [DOI] [PubMed] [Google Scholar]
- 25.Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev. 2006;25(4):521-529. doi: 10.1007/s10555-006-9036-9 [DOI] [PubMed] [Google Scholar]
- 26.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8(12):1369-1375. doi: 10.1038/ncb1507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Chemotherapy dose modification
eAppendix 2. Intensity-modulated radiation therapy (IMRT) planning protocol in this trial
eFigure 1. Median relative dose intensity of cisplatin and 5-fluorouracil in each cycle
eFigure 2. Forest plots of treatment effects on Overall Survival within subgroups
eTable 1. Chemotherapy and local treatment in primary metastasized malignancies
eTable 2. Chemotherapy plus local radiotherapy in metastatic NPC
Table 3. Distribution of patients by site of enrollment
Table 4. Members of the Independent Data Monitoring Committee (IDMC)
Table 5. Members of the ethics committee of SYSUCC
Table 6. Treatment exposure in the intention-to-treat population
Table 7. Disease recurrence distribution in the two treatment groups
Table 8. Summary of subsequent therapies
Data sharing statement