Scheme 4. Synthesis of Intermediates 43, 43a and 47a.
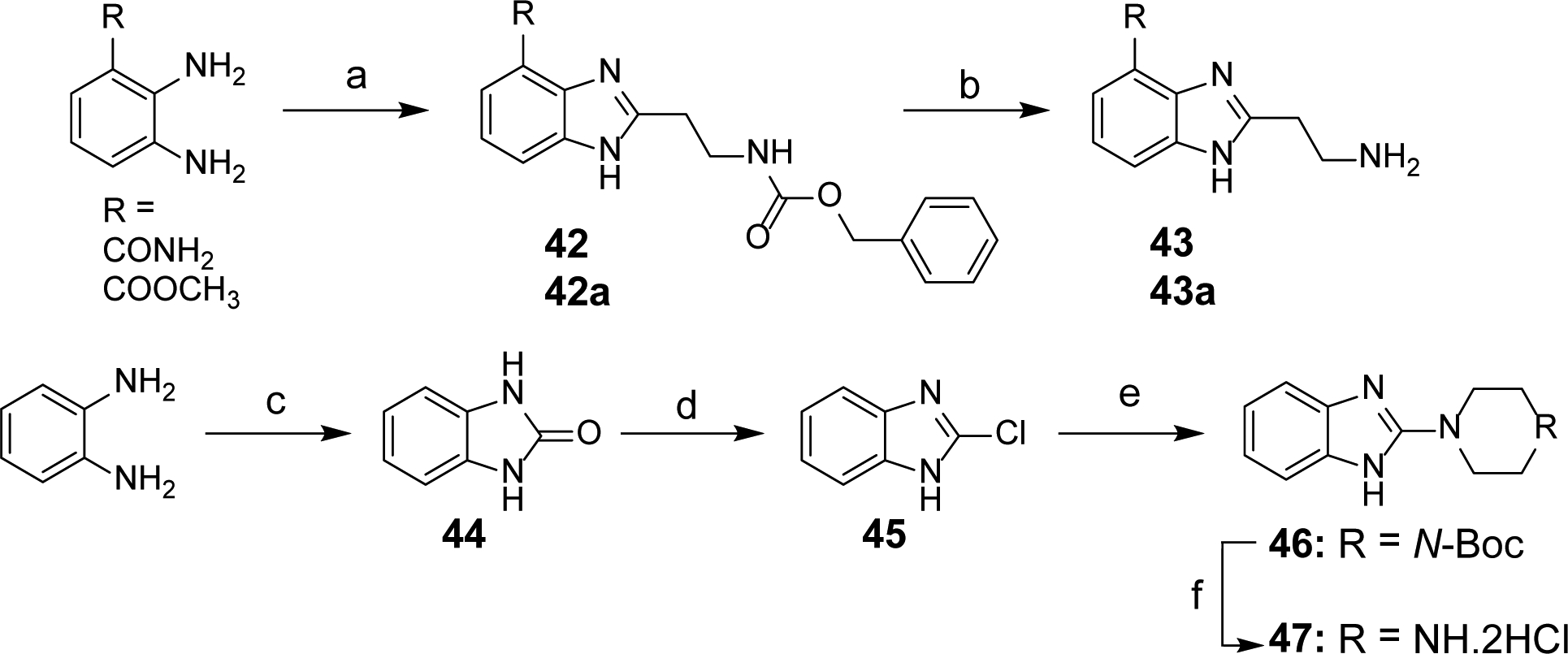
aReagents and conditions: (a) Benzyl 3-oxopropylcarbamate, NH4OAc, DMF, 80°C, 6 h (for 42); benzyl 3-oxopropylcarbamate, HCTU, EtN(i-Pr)2, DMF, rt to reflux, 10 h (for 42a); (b) H2, Pd/C, CH3OH, rt, 5 h; (c) 1,1′-carbonyldiimidazole, THF, rt, 22 h; (d) POCl3, 95°C, 16 h; (e) N-Boc piperazine, toluene, MW, 150°C, 6h; (f) 4N HCl, dioxane, rt, overnight.
