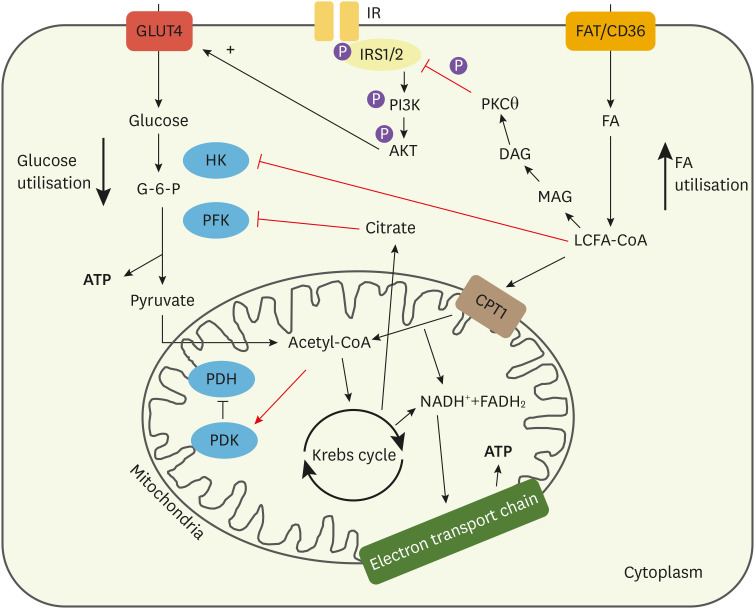
Fig. 2. Cellular mechanisms that favour FA use within the diabetic cardiomyocyte. Intermediates from FA breakdown inhibit components of glucose metabolism. LCFA-CoA can inhibit HK, the primary enzyme involved in glucose breakdown. Acetyl-CoA from increased FA oxidation can also activate PDK, the inhibitor of PDH. This subsequently reduces PDH activity, reducing pyruvate metabolism. Citrate from the Krebs cycle generated by increased FA metabolism can also inhibit PFK in glycolysis. Overall, this reduces glycolytic flux in the cardiomyocyte. Increased LCFA-CoA leads to increased DAG accumulation, which contributes to altered signalling and increased storage of fats as triglycerides. DAG can activate PKCθ, which has been suggested as the enzyme driving lipid-induced insulin resistance. It has been proposed that PKCθ can phosphorylate serine residues on the insulin receptor and its adaptor protein, IRS1/2. This prevents tyrosine phosphorylation, which is necessary for signalling, reducing translocation of vesicles containing GLUT4 to the membrane, reducing insulin-stimulated glucose uptake.
FA, fatty acids; LCFA-CoA, long chain fatty acyl coenzyme A; HK, hexokinase; PDK, pyruvate dehydrogenase kinase; PDH, pyruvate dehydrogenase; PFK, phosphofructokinase; DAG, diacylglycerol; PKCθ, protein kinase C theta; IRS1/2, insulin receptor substrates 1/2; GLUT, glucose transporter; G-6-P, glucose-6-phosphate.