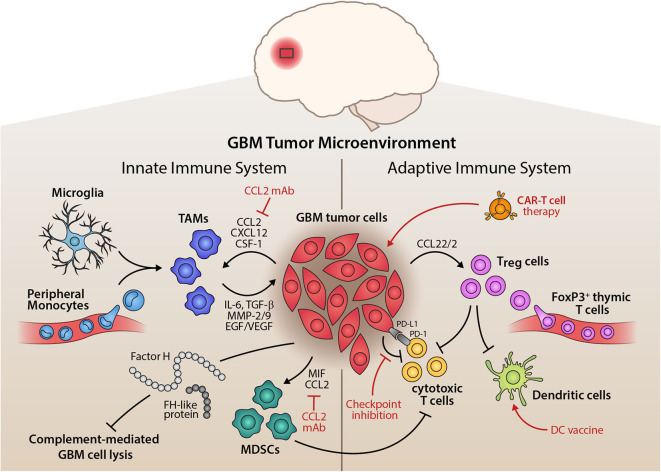
Figure 3.
Inflammatory Tumor Microenvironment of GBM and its Therapeutic Implications. Illustration of the interplay of innate and adaptive immune components within the glioma microenvironment. On the side of the innate immune system, tumor-associated macrophages (TAMs), mainly comprised of microglia and peripheral monocytes, are attracted by tumor cells, which release pro-inflammatory cytokines, matrix remodelers, and growth factors to aid tumorigenesis. Myeloid-derived suppressor cells (MDSCs) are also recruited by the tumor and potently suppress anti-tumor immunity. Alternative pathway molecules factor H (FH) and FH-like protein 1 of the complement system enhance immunosuppression and prevent complement-mediated lysis of the tumor cells. The adaptive immune system, on the other hand, is largely suppressed in its function through the recruitment of regulatory T cells (Treg). These inhibit the action of cytotoxic T cells and dendritic cells, disturbing a competent anti-tumor immune response. Tumor cells also exert direct suppression of adaptive immunity through immune checkpoint expression, e.g., PD-L1 or CTLA-4. Therapeutically, this tumor-immune crosstalk can be targeted by inhibiting chemoattractants of pro-tumor immune cells, such as anti-CCL2 monoclonal antibody, by immune checkpoint inhibition, dendritic cell vaccination approaches or adoptive transfer of chimeric antigen receptor (CAR) T cells that target the glioma cells (red indicators).