Abstract
Cholesterol is an essential molecule for life. It is a component of the cell membrane, and it is a precursor molecule for bile acids, vitamin D and steroid hormones. Cholesterol is actively metabolized, but the impact of endogenous cholesterol metabolites on immune function, especially in the intestine, is poorly understood. In this review, I focus on oxysterols, hydroxylated forms of cholesterol, and their specialized functions in intestinal immunity. Oxysterols act through various intracellular and extracellular receptors and serve as key metabolic signals, coordinating immune activity and inflammation. Our recent work has identified an unexpected link between cholesterol metabolism, innate lymphoid cell function and intestinal homeostasis. We discovered that oxysterol sensing through the G protein‐coupled receptor 183 (GPR183) directs the migration of innate lymphoid cells, which is essential for the formation of lymphoid tissue in the colon. Moreover, we found that the interaction of GPR183 with oxysterols regulates intestinal inflammation. I will discuss the therapeutic potential of oxysterols and future possibilities of treating inflammatory bowel disease through the modulation of cholesterol metabolism.
Keywords: cholesterol metabolism, inflammatory bowel disease, innate lymphoid cells, oxysterols
Introduction
Biosynthesis of oxysterols from cholesterol
Oxysterols are hydroxylated metabolites of cholesterol that can be generated enzymatically and nonenzymatically 1, 2. Oxysterols were first identified as intermediates in bile acid metabolism, but it is becoming clear that oxysterols have pleiotropic roles in immunity and inflammation 3, 4, 5, 6. Structurally distinct oxysterols that have immune activity are generated from cholesterol through the action of specific enzymes (Fig. 1). Cholesterol 25‐hydroxylase (CH25H) converts cholesterol into 25‐hydroxycholesterol (25‐OHC), which can be further metabolized to 7α,25‐dihydroxycholesterol (7α,25‐OHC) by the enzyme oxysterol 7α‐hydroxylase (CYP7B1). Another pathway leads to the synthesis of 27‐hydroxycholesterol (27‐OHC) from cholesterol by the enzyme mitochondrial sterol 27‐hydroxylase (CYP27A1). CYP7B1 then converts 27‐OHC into 7α,27‐dihydroxycholesterol (7α,27‐OHC). Both 7α,25‐OHC and 7α,27‐OHC are further metabolized by the enzyme 3β‐hydroxysteroid dehydrogenase type 7 (HSD3B7) into bile acid precursors.
Figure 1.
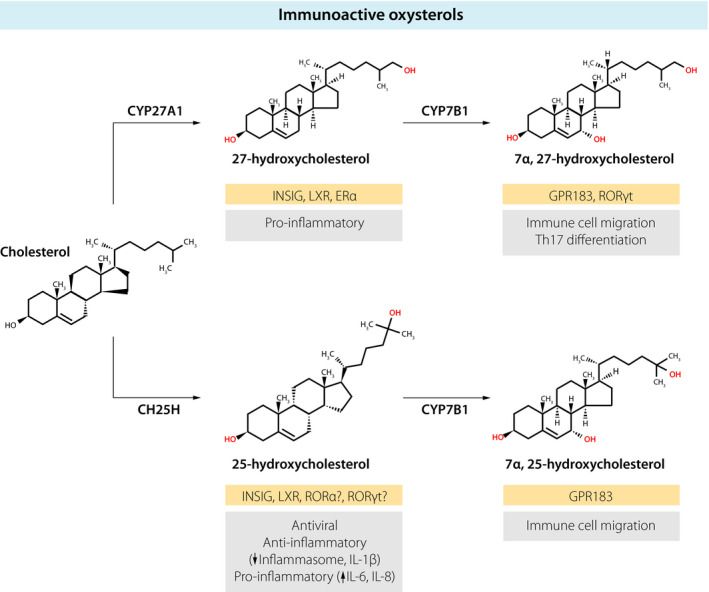
Immunologically active oxysterols. The biosynthesis of oxysterols from cholesterol through the action of specific enzymes (CH25H, CYP27A1, CYP7B1) is shown. Receptors for the different oxysterols are highlighted in gold boxes. The immunological functions of oxysterols are indicated in grey boxes.
Regulation of cholesterol homeostasis by oxysterols
Apart from serving as precursors for bile acid synthesis, oxysterols also regulate cholesterol homeostasis by binding to the transcription factors sterol regulatory element‐binding protein (SREBP) and liver X receptor (LXR) 2, 4. SREBP activates the expression of genes that promote cholesterol uptake and biosynthesis, whereas LXR induces genes that stimulate the removal of cholesterol. One target gene of SREBP is 3‐hydroxy‐3‐methylglutaryl‐CoA (HMG‐CoA) reductase, the key rate‐limiting enzyme in cholesterol synthesis. Monohydroxylated oxysterols derived from cholesterol (such as 25‐OHC and 27‐OHC) serve as activators of LXR and inhibitors of SREBP, thereby forming a negative feedback loop that is induced by excess cholesterol. 25‐OHC binding to insulin‐induced gene (INSIG) leads to formation of a protein complex consisting of INSIG, SREBP cleavage‐activating protein (SCAP) and SREBP, thereby sequestering SREBP and ultimately suppressing cholesterol biosynthesis.
Immune activity of oxysterols
Cells of the immune system have the ability to recognize different oxysterols by using various intra‐ and extracellular receptors. Immune cells express ‘generic’ oxysterol receptors, such as LXR, as well as receptors specifically expressed by immune cells, such as G protein‐coupled receptor 183 (GPR183), also known as Epstein–Barr virus‐induced gene 2 (EBI2). Consequently, oxysterols control several aspects of immune function that include cell migration, cytokine production, T helper cell differentiation, lymphocyte proliferation, macrophage phagocytosis, inflammasome activation and antiviral activity 3, 4, 5, 6. Here, I focus on the immunomodulatory function of monohydroxylated (25‐OHC, 27‐OHC) and dihydroxylated (7α,25‐OHC, 7α,27‐OHC) forms of cholesterol.
25‐hydroxycholesterol
25‐OHC can bind to many intracellular receptors in vitro, including INSIG, LXR and members of the retinoic acid receptor‐related orphan receptor (ROR) transcription factor family. For example, 25‐OHC has been proposed to be an inverse agonist for RORα 7 and to be an endogenous ligand for RORγt 8, 9. However, it is unclear which 25‐OHC receptors mediate its immune effects in vivo. In fact, there is limited evidence that 25‐OHC functions through LXR in vivo.
A number of recent studies have discovered that 25‐OHC acts as a potent endogenous antiviral agent 10, 11, 12, 13. Cholesterol is an integral part of the plasma membrane as well as of intracellular lipid compartments. Viruses require these lipid membranes for their life cycle and hijack the cholesterol metabolism of the host to facilitate their replication. It has been demonstrated that viral infection induces expression of CH25H in macrophages through type I interferons, resulting in 25‐OHC production. 25‐OHC inhibits viral entry, assembly and replication, likely through effects on the cholesterol content of the plasma membrane and intracellular membrane compartments.
Inflammatory stimuli, such as endotoxin administration, markedly increase the blood concentration of 25‐OHC in mice and humans 14, 15, and, in addition to its antiviral activity, 25‐OHC controls inflammatory responses. In regard to its anti‐inflammatory function, two studies by Cyster et al. have uncovered an unexpected role for 25‐OHC and cholesterol metabolism in regulating the activity of the inflammasome, a multiprotein complex that stimulates the production of interleukin‐1 (IL‐1) family cytokines. His laboratory reported that excess amounts of intracellular cholesterol trigger inflammasome activation in macrophages and IL‐1β secretion 16. Furthermore, experiments demonstrated that 25‐OHC suppresses inflammasome activation and IL‐1β production by repressing SREBP and therefore cholesterol synthesis 17. Thus, 25‐OHC mediates type I interferon‐induced suppression of inflammasome activation. In this scenario, viral infection induces type I interferon, which leads to CH25H upregulation and subsequent 25‐OHC synthesis, ultimately limiting IL‐1β‐mediated inflammation. Conversely, a pro‐inflammatory function for 25‐OHC has also been described in macrophages 18 and epithelial cells 19. In these studies, 25‐OHC stimulated the production of the pro‐inflammatory cytokines IL‐6 and IL‐8 as well as macrophage colony‐stimulating factor (M‐CSF). Consistent with a pro‐inflammatory role of 25‐OHC, mice deficient in CH25H had less inflammation‐induced lung damage after influenza infection 18. Overall, 25‐OHC may adjust the inflammatory response to provide optimal host defence, whilst limiting excessive inflammation and tissue injury.
27‐hydroxycholesterol
In steady state, 27‐OHC is the most abundant oxysterol in the blood 1, yet its immune activity is not well‐defined. 27‐OHC binds to INSIG, LXR and oestrogen receptor alpha (ERα). In general, LXR has anti‐inflammatory function 3, but similar to 25‐OHC it is unclear whether ligation of LXR by 27‐OHC has physiological significance in vivo. One study has reported that binding of 27‐OHC to ERα stimulates the pro‐inflammatory response of macrophages in atherosclerosis 20. More studies are needed to further elucidate the direct effects of 27‐OHC on the immune system and inflammatory responses.
7α,25‐dihydroxycholesterol
Dihydroxylated forms of cholesterol (7α,25‐OHC, 7α,27‐OHC) act through the cell surface receptor GPR183 and function as chemotactic cues for immune cells. Pioneering studies by the Cyster and Brink groups demonstrated that GPR183 is required for the proper positioning of B lymphocytes in the spleen, thereby promoting antibody production during an immune response 21, 22. Subsequently, it was discovered that oxysterols are ligands for GPR183, with 7α,25‐OHC being the most potent one 23, 24. Genetic and pharmacological disruption of 7α,25‐OHC synthesis confirmed that the interaction of 7α,25‐OHC with its receptor GPR183 is critical for B‐cell localization in the spleen and antibody responses 23, 24, 25. Subsequent work identified a role for GPR183 and 7α,25‐OHC in the homeostasis of dendritic cells by directing their migration to a specific niche in the spleen 26, 27. Similarly, another study showed that GPR183 guides the migration of activated T lymphocytes within the spleen, which promotes their differentiation to T follicular helper cells 28.
7α,27‐dihydroxycholesterol
Recently, in vivo GPR183 ligand activity has also been reported for 7α,27‐OHC 29, although in vitro it is 10‐fold less potent than 7α,25‐OHC 23, 24. Disruption of 7α,27‐OHC production in Cyp27a1 knockout mice causes altered dendritic cell migration and impaired dendritic cell maintenance 29. 7α,27‐OHC (and 7β,27‐OHC) has also been proposed to be an endogenous RORγt agonist, thereby supporting the differentiation of T helper 17 (Th17) cells 9. Further studies are needed to verify this interesting observation.
Intestinal lymphoid tissues
Innate lymphoid cells
Innate lymphoid cells (ILCs) are an emerging family of immune cells that are enriched in tissues interacting with the outside world, such as the intestine, lung and skin. ILCs play an important role in organ homeostasis, tissue repair, metabolism and host defence 30, 31. Apart from cytotoxic natural killer cells, three different groups of ILCs can be distinguished based on expression of signature transcription factors and effector cytokines, similar to CD4+ T helper lymphocytes. Group 3 ILCs (ILC3s) are characterized by expression of the transcription factor RORγt and produce the cytokines IL‐17 and IL‐22. ILC3s can be further divided into two subsets that differ in their ontogeny, localization and function 32. One of these subsets consists of foetal lymphoid tissue inducer (LTi) and adult LTi‐like ILC3s (CCR6+) that have the specialized function of orchestrating the formation of lymphoid tissues.
Intestinal lymphoid tissues in mice and humans
Lymphoid tissues are important communication centres of the immune system. The intestine is particularly rich in lymphoid tissues, which not only include mesenteric lymph nodes, but also Peyer's patches/colonic patches as well as smaller, so‐called solitary lymphoid tissues (SILT), namely cryptopatches and isolated lymphoid follicles 33, 34. These structures have an essential function in maintaining the symbiosis between the host and the gut microbiota, for example through the T‐cell‐independent production of immunoglobulin A (IgA). Cryptopatches consist mainly of ILC3s surrounded by a ring of dendritic cells within a network of stromal cells. The formation of cryptopatches is genetically programmed, and, in mice, they develop within three weeks after birth. The recruitment of B cells to cryptopatches leads to the formation of isolated lymphoid follicles.
Gut‐associated lymphoid tissue in humans consists of Peyer's patches, the appendix and scattered solitary lymphoid follicles 35. Due to experimental limitations, lymphoid tissue formation in humans, especially in the intestine, is much less well understood than in mice. It has been reported that lymphoid structures in the small intestine differ between humans and mice 36 and that the human intestine lacks cryptopatches 37. In contrast, another study reported that isolated lymphoid follicles are present in the normal human colon 38. The discrepancies between the studies may be due to technical issues, that is the antibody staining to define cryptopatches. Consistent with this possibility, another study argued that c‐kit expression, which is often used to identify ILC3s and thereby cryptopatches in mice, is not suited for this purpose in the human intestine. The authors showed that CCR6+ clusters resembling murine cryptopatches can be detected in the human intestine 39. Importantly, these clusters contained RORγt+CCR6+ cells with intermediate c‐kit expression, which likely defines them as human LTi‐like ILC3s. Finally, one study using a chimeric human–mouse model demonstrated that mouse cryptopatches can act as precursors for larger lymphoid follicles containing human immune cells 40. Taken together, these studies indicate that SILT formation is likely to be conserved between humans and mice.
Role of intestinal lymphoid tissue in colitis
Chronically inflamed tissue resembles organized lymphoid tissue and mouse studies have shown that increased lymphoid tissue formation correlates with the severity of intestinal inflammation, although a direct causal role is still being debated 34, 41, 42. There is also evidence that lymphoid tissues may contribute to the disease pathology in human inflammatory bowel disease (IBD) 43. Basal lymphoid aggregates in the inflamed intestine are a distinctive feature of IBD, and they are already present at disease onset 44, 45. Hyperplastic lymphoid aggregates in ulcerative colitis 35 contain CCR6+ LTi‐like ILC3s 39, and the size of these aberrant aggregates correlates with disease severity 38. It is unknown whether lymphoid aggregates in IBD develop from pre‐existing secondary lymphoid structures or, alternatively, whether they represent newly formed tertiary inflammatory lymphoid tissue. Overall, it seems reasonable to infer that these lymphoid structures have a pro‐inflammatory function in IBD, sustaining chronic tissue damage.
The cytokine tumour necrosis factor alpha (TNFα) plays a role in chronic intestinal inflammation 43, and, accordingly, TNFα neutralization using antibody administration has been an effective treatment in a subgroup of IBD patients 46. Interestingly, TNFα and members of the TNF superfamily play a role in lymphoid organogenesis in mice. It has been proposed that TNFα drives the development of tertiary lymphoid structures in the inflamed intestine 47. It is therefore possible that some of the therapeutic effects of anti‐TNFα treatment in IBD is due to the suppression of lymphoid tissue neogenesis in the inflamed intestine.
Development of intestinal lymphoid tissues
In mice, LTi cells establish tissue residency in the intestine early during development, which is linked to their function of promoting the formation of lymphoid tissue in anticipation of the colonization of the intestine with the commensal microbiota. In general, lymphoid tissue formation is critically dependent on ILC3s that produce lymphotoxin. Beyond this general requirement, the specific factors regulating SILT formation have been identified in the small intestine. These include RANKL, CCL20, CXCL13 (and their corresponding receptors RANK, CCR6, CXCR5) and the gut microbiota 48, 49, 50, 51. In contrast, the microbiota and the other signals essential for SILT formation in the small intestine (RANKL, CCL20, CXCL13) are not required in the colon 52. Therefore, the specific signals required for lymphoid tissue formation in the colon have remained unknown until recently.
A novel role for the oxysterol receptor GPR183 in intestinal immunity
In the intestine, ILCs are exposed to an environment that is rich in microbe‐ and diet‐derived factors as well as metabolites. Accordingly, ILCs have to respond appropriately to environmental challenges to carry out their task as sentinels of healthy tissue function. This requires that (i) ILCs express receptors allowing them to detect environmental signals and that (ii) ILCs are strategically positioned within the tissue. However, it is poorly understood how ILCs directly sense cues from the local environment and which signals regulate their migration within the intestine.
Sensing of environmental signals by intestinal ILCs
ILCs lack classical antigen receptors, but it is becoming clear that ILCs can directly sense environmental signals through the expression of receptors that detect metabolic, microbial and neuronal cues. Apart from antigen‐specific receptors, immune cells use other receptor families for environmental sensing. Metabolites can be detected through intracellular receptors, such as nuclear receptors that act as transcription factors, or, alternatively, through extracellular receptors. A prominent example of the latter is G protein‐coupled receptors (GPCRs), an important class of cell surface receptors with key functions in many organ systems. GPCRs mediate sensing of environmental signals and regulate cell migration in the immune system. We therefore hypothesized that ILCs are regulated by GPCRs that recognize metabolites. This drew our attention to GPR183, and, given the role of GPR183 in immune cell migration, we reasoned that oxysterols could serve as guidance cues for ILCs and regulate their function in the intestine 53.
GPR183 orchestrates ILC migration and lymphoid tissue formation in the colon
Consistent with our hypothesis, GPR183 is highly expressed by LTi‐like ILC3s and ILC3s migrate towards 7α,25‐OHC in vitro 53. Furthermore, we found that GPR183 and its ligand 7α,25‐OHC promote ILC3 migration to cryptopatches and isolated lymphoid follicles in the colon 53. Using mice lacking either the receptor GPR183 in ILC3s or the enzyme CH25H that is critical for oxysterol synthesis from cholesterol demonstrated that GPR183 and 7α,25‐OHC are essential for lymphoid tissue formation in the colon 53. In contrast, they are not required for the development of lymph nodes and Peyer's patches. Interestingly, in the small intestine, cryptopatches and isolated lymphoid follicles form in the absence of either GPR183 or 7α,25‐OHC 53, despite the observation that GPR183 and 7α,25‐OHC‐synthesizing enzymes are expressed in both colon and small intestine. Moreover, 7α,25‐OHC attracts ILC3s to cryptopatches not only in the colon, but also in the small intestine. Most likely B cells acquire compensatory LTi activity when ILC3 migration to cryptopatches is impaired. This is supported by our finding that enlarged isolated lymphoid structures consisting of B cells form in the small intestine of CH25H‐deficient mice 53. It is therefore plausible that lymphoid tissue formation in the small intestine is rescued because microbiota‐induced chemokines, such as CCL20 and CXCL13, recruit B cells to isolated lymphoid follicles. Thus, redundant factors in the small intestine likely compensate for the lack of oxysterols and GPR183.
Localized, microbiota‐independent, 7α,25‐OHC production occurs in colonic lymphoid structures 53. This is mediated by specialized podoplanin+CD34−CXCL13+stromal cells residing in cryptopatches and isolated lymphoid follicles 53. Based on their phenotype, they can be classified as fibroblastic stromal cells. A second subset of podoplanin+ stromal cells mainly resides outside of cryptopatches and isolated lymphoid follicles. This population is CD34+ and highly expresses the enzyme HSD3B7 that degrades the GPR183 ligand 7α,25‐OHC 53. Therefore, these stromal cells likely act as a sink for 7α,25‐OHC produced by podoplanin+CD34−CXCL13+stromal cells, thereby creating an oxysterol gradient that attracts GPR183+ ILCs to the sites where cryptopatches are formed.
Previous work indicated that oxysterols exert complex effects on IgA production in the intestine. It has been reported that 25‐OHC suppresses IgA production through inhibiting class switching in vitro 14. Accordingly, CH25H‐deficient mice have lower amounts of IgA 14. In contrast, IgA is moderately increased in mice lacking CYP7B1 14, suggesting that 7α,25‐OHC‐mediated B‐cell positioning may stimulate IgA generation. However, we found that GPR183 deficiency does not significantly alter intestinal IgA concentrations 53.
Implications
Our discovery that ILC3s sense oxysterols through the surface receptor GPR183 has identified a new mechanism of how ILCs directly detect environmental signals. In this case, the oxysterols 7α,25‐OHC and 7α,27‐OHC are sensed through a cell surface receptor, which regulates cell migration and tissue remodelling in steady state and inflammation (Fig. 2). Overall, these findings suggest that ILCs have the ability to sense changes in host cholesterol metabolism. A recent study confirmed our results, in addition reporting that GPR183 is required for ILC3‐mediated immunity against intestinal bacteria 54. An interesting area for future research will be to determine how ILCs and other immune cells integrate signals by chemically distinct oxysterols that are encountered within the same tissue compartment.
Figure 2.
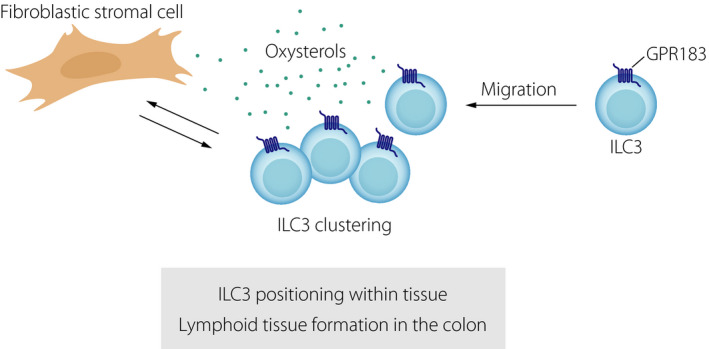
The oxysterol receptor GPR183 orchestrates ILC migration, lymphoid tissue formation and inflammatory responses in the colon. Oxysterols produced by fibroblastic stromal cells attract GPR183‐expressing ILCs to sites where lymphoid tissues (cryptopatches, isolated lymphoid follicles) are formed in the steady‐state colon. The oxysterol‐GPR183 pathway also controls the formation of inflammatory infiltrates during colitis.
Moreover, our study provides information on the mechanisms that control the spatial and functional compartmentalization of ILC3s in the intestine. Recognition of oxysterol guidance cues by GPR183 directs LTi‐like ILC3s to cryptopatches, which promotes their lymphoid tissue‐inducing function 53. This complements previous work showing that CXCL16 guides the positioning of CXCR6+ NKp46+ ILC3s to the villi of the small intestine, where they contribute to epithelial defence through the production of IL‐22 55. In mesenteric lymph nodes, ILC3s are also found in a specific anatomical location, namely the interfollicular region 56 and this ILC3 positioning in the interfollicular region is also dependent on GPR183 (our unpublished data). Taken together, our work demonstrates that oxysterols control ILC localization and function in lymphoid tissues by engaging the receptor GPR183.
Metabolic control of lymphoid tissue formation in the intestine
Our finding that oxysterols recognized through GPR183 act as a major signal for lymphoid tissue formation in the colon supports the emerging link between metabolism, lymphoid tissue formation and immune fitness. Previous work has demonstrated that intestinal ILCs respond to diet‐derived metabolites, which control lymphoid tissue organogenesis. For example, it was shown that maternal amounts of the vitamin A metabolite retinoic acid control the size of secondary lymphoid tissues in the offspring through promoting the differentiation of foetal LTi cells, which determines antiviral immunity later in life 57. Conversely, lack of vitamin A in adult mice, as it occurs in malnutrition, leads to impaired lymphoid tissue formation in the small intestine 58. Moreover, expression of the Aryl hydrocarbon receptor (AHR) allows intestinal ILC3s to respond to phytochemicals derived from vegetables, such as broccoli, that have AHR ligand activity. This process is required for the postnatal expansion of ILC3s and, consequently, for the formation of cryptopatches and isolated lymphoid follicles in the intestine 59, 60.
Our finding that oxysterols promote the formation of lymphoid tissue in the colon raises the interesting question about the source of cholesterol that is used for oxysterol synthesis in the intestine – specifically whether oxysterols are derived from intracellular cholesterol or from cholesterol taken up with the diet. The latter would suggest a possible link between dietary cholesterol intake and lymphoid tissue formation in the colon. This possibility is supported by the observation that, in mice, cryptopatches develop within the first 3 weeks after birth during the period of breastfeeding 49. It is therefore tempting to speculate that cholesterol contained in breast milk is converted into oxysterols that induce colonic lymphoid structures.
Overall, mice lacking nutrients (vitamin A, phytochemicals, oxysterols) during the perinatal period exhibit defects in intestinal lymphoid tissue formation. This link may have evolved to balance nutrient availability (i.e. dietary input) with intestinal immunity. The perinatal period is a critical time when maturation of the immune system occurs and when the microbiota colonizes the intestine. It has therefore a potentially large impact on immune responses later in life and the susceptibility to develop chronic inflammatory diseases.
Oxysterols in intestinal inflammation
GPR183 controls inflammatory tissue remodelling in colitis
Chronic inflammation in IBD is thought to be caused by a dysregulated immune response against the host's own microbiota (Fig. 3), which is characterized by the activation of pro‐inflammatory immune activity and subsequent tissue damage 61. The inflammatory response results in tissue infiltration by recruited immune cells (such as myeloid cells) and a profound tissue reorganization. Immune cells residing in lymphoid tissues participate in the inflammatory process and likely contribute to colitis. Accordingly, it has been shown that cryptopatch‐resident ILC3s are not sessile, but actively migrate in and out of cryptopatches 62. During inflammation, ILC3 egress from cryptopatches is increased leading to their mobilization into the surrounding tissue. ILC3‐derived cytokines, such as granulocyte macrophage colony‐stimulating factor (GM‐CSF), then lead to the recruitment of circulating inflammatory monocytes to the colon 62. Given that GPR183 and its oxysterol ligands promote ILC3 migration and lymphoid tissue organogenesis in the colon, we reasoned that this pathway is not only active in the steady state, but also during inflammation‐induced tissue remodelling. This was confirmed in a mouse model of innate immune‐mediated colitis 53. Colon inflammation increased the production of GPR183 ligands through the upregulation of the ligand‐synthesizing enzyme CH25H combined with the downregulation of the ligand‐degrading enzyme HSD3B7 53. Increased oxysterol production coincided with the recruitment of GPR183‐expressing ILCs and myeloid cells to inflammatory foci in the colon. Furthermore, colitis severity was reduced in mice lacking the GPR183 receptor in innate immune cells 53. In support of our findings, another group reported that chemical disruption of the intestinal barrier causes increased 7α,25‐OHC production in the inflamed colon, which correlated with colitis severity 63. Altogether, these findings demonstrate that GPR183 and oxysterols support ILC3‐mediated tissue remodelling in the inflamed intestine, which is reminiscent of the lymphoid tissue‐inducing function of ILC3s during development (Fig. 2). It is reasonable to assume that increased oxysterol synthesis induced by tissue injury conveys perturbation of tissue homeostasis to the immune system, initiating the inflammatory response.
Figure 3.
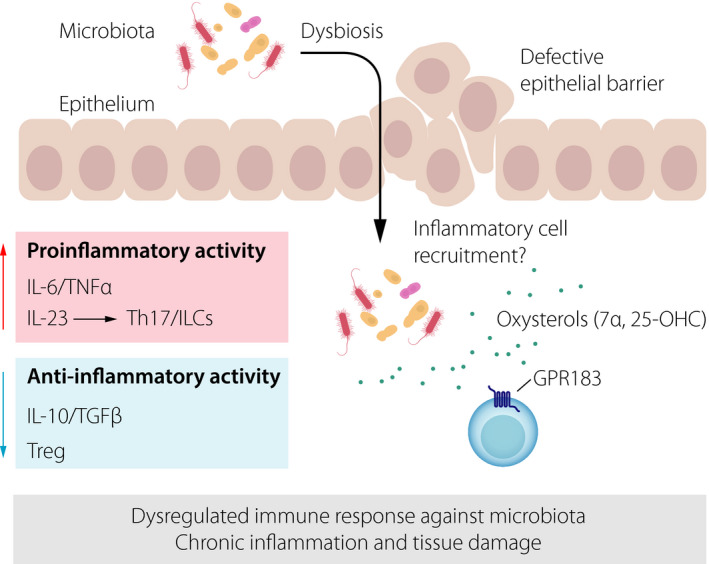
Key mechanism of intestinal inflammation. Defective epithelial barrier function combined with dysbiosis stimulates pro‐inflammatory immune activity, whilst anti‐inflammatory pathways are impaired. This leads to a dysregulated immune response against the host's microbiota, thereby causing chronic inflammation and tissue damage. The possible role of oxysterols (sensed through the receptor GPR183 on immune cells) in promoting inflammatory cell recruitment is shown.
Importantly, the GPR183‐7α,25‐OHC pathway is conserved in humans and we have found that CH25H and CYP7B1 expression correlates with the degree of local inflammation in paediatric patients with ulcerative colitis 53, suggesting a role for oxysterols and their receptor GPR183 in human IBD (Fig. 4). This finding is in line with other studies that have reported upregulation of the enzymes producing the GPR183 ligand 7α,25‐OHC in chronic inflammatory and autoimmune diseases. Furthermore, CH25H‐dependent oxysterols and GPR183 promote the recruitment of activated T lymphocytes to the inflamed central nervous system 64, indicating a general role of this pathway in inflammatory cell recruitment. Further studies are needed regarding the role of GPR183‐expressing immune cells in other mouse models of colitis, where adaptive immune cells are important.
Figure 4.
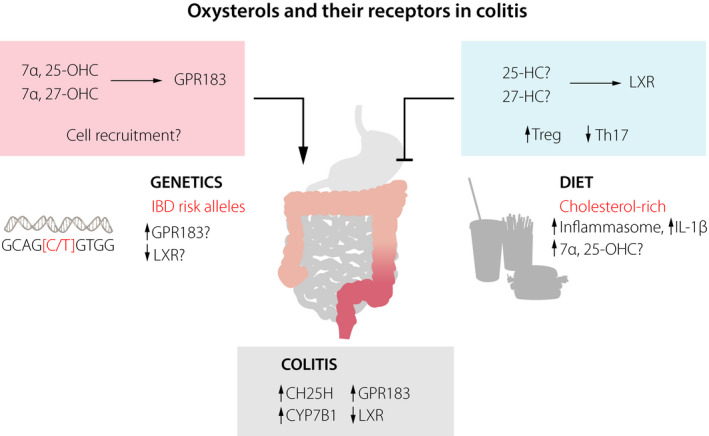
Role of oxysterols and their receptors in colitis. The expression of oxysterol‐synthesizing enzymes (CH25H, CYP7B1) and GPR183 is increased in the inflamed colon, whilst expression of LXR is decreased. Recognition of oxysterols by the receptor GPR183 likely promotes colitis through immune cell recruitment, whereas the receptor LXR inhibits colitis through regulation of T helper cells. Polymorphisms in GPR183 and LXR have been associated with IBD and could affect their expression. A cholesterol‐rich diet could predispose to IBD by activating the inflammasome and/or increasing amounts of the GPR183 ligand 7α,25‐OHC.
Role of other oxysterols/oxysterol receptors in colitis
LXR has mainly been attributed to have anti‐inflammatory activity 3. Accordingly, it has been shown that LXR‐deficient mice are more susceptible to chemically induced colitis 65. This study also reported lower LXR expression in the inflamed colon of IBD patients. LXR ligation by oxysterols may protect from colitis through the induction of intestinal regulatory T cells 66, whilst inhibiting the differentiation of pro‐inflammatory Th17 cells 67. However, it is unclear which oxysterol is the relevant LXR ligand in the context of intestinal inflammation. The potential role of other oxysterols/oxysterol receptors (apart from GPR183 and LXR) in colitis needs further investigation (Fig. 4).
Genetic variation in oxysterol receptors and IBD
A single nucleotide polymorphism (SNP) in the GPR183 gene was linked to type 1 diabetes 68, supporting the concept that genetic variation in this oxysterol receptor may be linked to chronic inflammatory responses. A subsequent genome‐wide association study identified the GPR183 locus as a risk allele for human IBD 69. The reported polymorphisms are in the noncoding region of the GPR183 gene and therefore likely affect GPR183 expression 68. However, their functional significance is unknown and requires further investigation. It is also unclear which immune cell type(s) is affected by these polymorphisms. It is interesting to speculate that natural genetic variation in GPR183 and GPR183 ligand‐synthesizing enzymes (CH25H, CYP7B1) could affect the size of intestinal lymphoid tissues in humans and the predisposition to develop intestinal inflammation (Fig. 4). SNPs in the gene encoding LXR are also associated with human IBD 70, and, similar to GPR183, these polymorphisms warrant further exploration.
A link between dietary cholesterol and colitis?
Oxysterols are synthesized from cholesterol, and, whilst cells have the ability to synthesize cholesterol themselves, it can also be taken up with the typical cholesterol‐rich Western diet. Therefore, it is interesting to explore whether a diet high in cholesterol is linked to IBD (Fig. 4). In this scenario, high amounts of oxysterols synthesized from diet‐derived cholesterol would activate the receptor GPR183 and trigger pro‐inflammatory immune activity in the intestine. This possibility is supported by the higher prevalence of IBD in Western societies 71. Moreover, a Western‐style diet rich in cholesterol is associated with an increased risk to develop IBD 72, suggesting that increased dietary intake of cholesterol predisposes to intestinal inflammation. Some experimental data in mice further support this concept. For example, a cholesterol‐rich diet stimulates the production of type I interferon by enterocytes, which results in increased CH25H expression and consequently 25‐OHC production 73. In addition, feeding mice a high cholesterol diet causes inflammasome activation in the gut epithelium, which induces IL‐1β production and subsequent recruitment of inflammatory myeloid cells 74. Finally, oxysterols, such as 7α‐OHC and 7β‐OHC, can be generated nonenzymatically through the auto‐oxidation of cholesterol present in food with a high cholesterol content. In this respect, it has been proposed that 7α‐OHC and 7β‐OHC negatively affect intestinal barrier function and induce pro‐inflammatory cytokine production 75.
Therapeutic potential of oxysterol modulation in IBD
Based on genetic studies and animal models, several anti‐inflammatory strategies have been developed to treat IBD 46, 61. Some of them are already in clinical use, such as anti‐TNFα agents and the α4β7 integrin blocker vedolizumab 46, whilst others are currently under evaluation in clinical trials (Table 1). However, not all IBD patients respond to a specific anti‐inflammatory treatment and therefore additional therapeutic approaches need to be explored. The discovery of oxysterols as a novel class of molecules controlling ILC function and tissue remodelling in the intestine indicates that influencing oxysterol synthesis and activity could have therapeutic value in IBD (Fig. 5).
Table 1.
Anti‐inflammatory treatment of IBD
| Target | Drug | Efficacy in IBD | Current status |
|---|---|---|---|
| Blockade of pro‐inflammatory cytokines | |||
| TNFα | Infliximab and others | Crohn's disease, ulcerative colitis | In clinical use |
| IL‐6 | Tocilizumab | Crohn's disease | In testing phase |
| IL‐12/IL‐23 | Ustekinumab and others | Crohn's disease | In clinical use |
| IL‐23 | Risankizumab | Crohn's disease | In testing phase |
| Inhibition of cytokine signalling | |||
| JAK1‐JAK3 | Tofacitinib | Ulcerative colitis | In testing phase |
| JAK1 | Filgotinib | Crohn's disease | In testing phase |
| PDE4 | Apremilast | Crohn's disease?, ulcerative colitis? | In testing phase |
| Inhibition of transcription factors | |||
| RORγt | NA | Crohn's disease?, ulcerative colitis? | Awaiting testing |
| GATA3 | NA | Ulcerative colitis? | Awaiting testing |
| Activation of anti‐inflammatory pathways | |||
| SMAD7 | Mongersen | Crohn's disease? | In testing phase |
| T regulatory cells | Cell therapy | Crohn's disease | In testing phase |
| Inhibition of T lymphocyte trafficking | |||
| α4β7 integrin | Vedolizumab | Crohn's disease, ulcerative colitis | In clinical use |
| β7 integrin | Etrolizumab | Ulcerative colitis | In testing phase |
| MAdCAM1 | PF‐00547659 | Ulcerative colitis | In testing phase |
| S1P receptors | Ozanimod and others | Ulcerative colitis | In testing phase |
Figure 5.
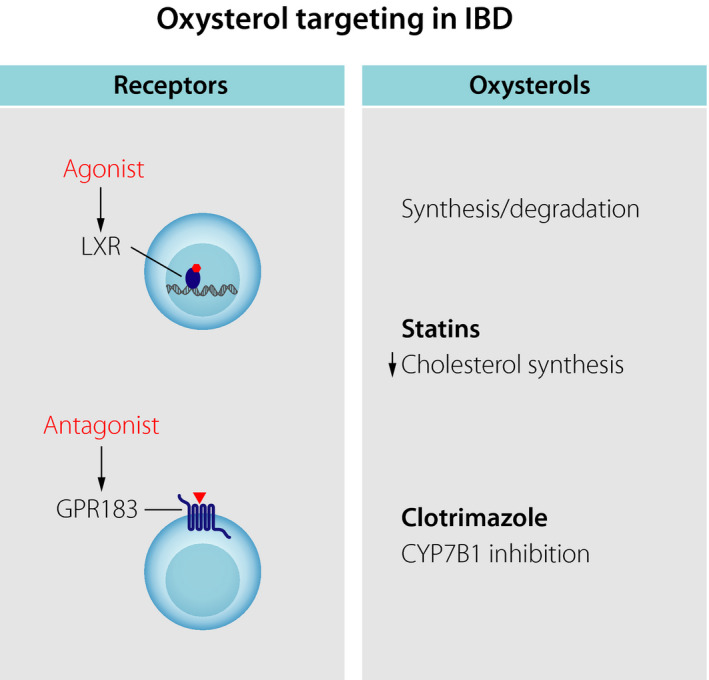
Possible therapeutic targeting of oxysterols in colitis. Small‐molecule agonists and antagonists could be used to inhibit the activity of the oxysterol receptors LXR and GPR183, respectively. Alternatively, drugs affecting the synthesis or degradation of oxysterols could be employed to modulate oxysterol function.
Targeting of oxysterols and their receptors
For this purpose, small molecules with agonist and antagonist activity towards the oxysterol receptors LXR and GPR183, respectively, could be used. Our findings indicate that GPR183 could represent a potential therapeutic target for IBD. This is an attractive possibility since GPCRs, such as GPR183, represent the largest family of cell surface receptors in the human genome and are the most common targets of medical drugs. Furthermore, GPR183 agonists and antagonists have already been developed to probe the function of GPR183 76, 77, 78. These small‐molecule agents could be further harnessed to modulate GPR183 activity in human disease. An LXR agonist had a protective effect in a mouse model of colitis 65. However, an unwanted side effect of current LXR agonists is the stimulation of fatty acid synthesis, causing hypertriglyceridaemia 79.
An alternative approach is to alter oxysterol concentrations in tissues through inhibiting their biosynthesis or degradation. In the case of 7α,25‐OHC, disrupting intratissue gradients by increasing or lowering the local 7α,25‐OHC concentration disrupts GPR183‐mediated cell migration 25. A few classes of drugs acting on cholesterol and oxysterols are available. For example, statins are widely used drugs that lower circulating cholesterol by inhibiting the key enzyme HMG‐CoA reductase. It is unclear whether the cholesterol content within tissue and cholesterol‐lowering drugs, such as statins, affect the immunomodulatory function of oxysterols. Statins mostly have anti‐inflammatory effects, which, however, have been suggested to be largely independent of their cholesterol‐lowering activity 3. Nevertheless, it has been reported that statin use is associated with a decreased risk to develop IBD 80. One drug targeting oxysterol production directly is the antifungal drug clotrimazole that inhibits CYP7B1 23 and has some therapeutic effect in rheumatoid arthritis 5. In a rat model of colitis, clotrimazole mitigated intestinal inflammation 81, yet further studies are required to assess the potential anti‐inflammatory role of clotrimazole in the intestine.
Challenges and future directions
When considering the therapeutic potential of oxysterol modulation, it is important to take into account the pleiotropic effects of different oxysterols acting through many receptors. Multiple receptors for specific oxysterols have been described in vitro, and it is important to clarify which receptor–oxysterol interactions are relevant in vivo. Furthermore, chemically distinct oxysterols likely coexist in the same tissue and may have multiple, even counteracting, effects on immune cells as well as epithelial cells in the intestine. Therefore, depending on cellular context and tissue, oxysterols may have pro‐ or anti‐inflammatory function. This is illustrated by the observation that dihydroxylated oxysterols acting through GPR183 support immune cell migration and inflammatory lymphoid tissue formation in the colon 53. Conversely, 25‐OHC limits inflammasome activity and therefore the production of pro‐inflammatory cytokines, such as IL‐1β 17. However, 25‐OHC has also been shown to have pro‐inflammatory function 18.
Another important area of investigation is the quantification and visualization of oxysterols in the intestine. The relevant in vivo concentrations of oxysterols which mediate their physiological effects are largely unknown and it will be critical to accurately quantify distinct oxysterols in the intestine, including in intestinal biopsies from humans with IBD. However, measuring lipids, such as oxysterols, in the intestine still represents a challenge. Currently, it is not possible to directly visualize oxysterol gradients that guide immune cell localization within the steady‐state intestine. Therefore, it is also unknown how tissue injury and inflammation impact these chemotactic oxysterol gradients.
Another related important area for future research is to determine the cellular source of oxysterols in the inflamed colon. As outline above, a specialized population of stromal cells residing in colonic lymphoid structures abundantly expresses the 7α,25‐OHC‐synthesizing enzymes CH25H and CYP7B1 in the steady state 53. Stromal cells have recently come into focus as a potential new cellular target in IBD. However, it is also possible that infiltrating myeloid cells provide a source of oxysterols, such as 7α,25‐OHC and 7α,27‐OHC, in colitis. This notion is supported by the observation that inflammatory signals, such as LPS, increase CH25H and CYP7B1 expression in macrophages and dendritic cells 14, 23.
Concluding remarks
Over the last few years, it has become evident that oxysterols exert profound effects on immune function. Our discovery linking cholesterol metabolism to ILC function, lymphoid tissue development and inflammatory responses in the intestine indicates that it is worth further exploring the role of the GPR183‐oxysterol pathway in the intestine and its potential therapeutic potential in human IBD.
Conflict of interest statement
No conflict of interest to declare.
Acknowledgements
I would like to thank for the support from the Center for Innovative Medicine (CIMED) at Karolinska Institutet (Junior Investigator Research Grant) funded by the Stockholm City Council and for the support by the Åke Wiberg Foundation.
Willinger T (Karolinska Institutet, Stockholm, Sweden). Oxysterols in intestinal immunity and inflammation (Review). J Intern Med 2019; 285: 367–380.
References
- 1. Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 2003; 72: 137–74. [DOI] [PubMed] [Google Scholar]
- 2. Luu W, Sharpe LJ, Capell‐Hattam I, Gelissen IC, Brown AJ. Oxysterols: old tale, new twists. Annu Rev Pharmacol Toxicol 2016; 56: 447–67. [DOI] [PubMed] [Google Scholar]
- 3. Spann NJ, Glass CK. Sterols and oxysterols in immune cell function. Nat Immunol 2013; 14: 893–900. [DOI] [PubMed] [Google Scholar]
- 4. Cyster JG, Dang EV, Reboldi A, Yi T. 25‐Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol 2014; 14: 731–43. [DOI] [PubMed] [Google Scholar]
- 5. Sun S, Liu C. 7alpha, 25‐dihydroxycholesterol‐mediated activation of EBI2 in immune regulation and diseases. Front Pharmacol 2015; 6: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fessler MB. The intracellular cholesterol landscape: dynamic integrator of the immune response. Trends Immunol 2016; 37: 819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tuong ZK, Lau P, Du X et al RORalpha and 25‐Hydroxycholesterol crosstalk regulates lipid droplet homeostasis in macrophages. PLoS ONE 2016; 11: e0147179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin L, Martynowski D, Zheng S, Wada T, Xie W, Li Y. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORgamma. Mol Endocrinol 2010; 24: 923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soroosh P, Wu J, Xue X et al Oxysterols are agonist ligands of RORgammat and drive Th17 cell differentiation. Proc Natl Acad Sci USA 2014; 111: 12163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu SY, Sanchez DJ, Aliyari R, Lu S, Cheng G. Systematic identification of type I and type II interferon‐induced antiviral factors. Proc Natl Acad Sci USA 2012; 109: 4239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blanc M, Hsieh WY, Robertson KA et al The transcription factor STAT‐1 couples macrophage synthesis of 25‐hydroxycholesterol to the interferon antiviral response. Immunity 2013; 38: 106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu SY, Aliyari R, Chikere K et al Interferon‐inducible cholesterol‐25‐hydroxylase broadly inhibits viral entry by production of 25‐hydroxycholesterol. Immunity 2013; 38: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li C, Deng YQ, Wang S et al 25‐Hydroxycholesterol protects host against zika virus infection and its associated microcephaly in a mouse model. Immunity 2017; 46: 446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bauman DR, Bitmansour AD, McDonald JG, Thompson BM, Liang G, Russell DW. 25‐Hydroxycholesterol secreted by macrophages in response to Toll‐like receptor activation suppresses immunoglobulin A production. Proc Natl Acad Sci USA 2009; 106: 16764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diczfalusy U, Olofsson KE, Carlsson AM et al Marked upregulation of cholesterol 25‐hydroxylase expression by lipopolysaccharide. J Lipid Res 2009; 50: 2258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dang EV, McDonald JG, Russell DW, Cyster JG. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell 2017; 171: 1057–71 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG. Inflammation. 25‐Hydroxycholesterol suppresses interleukin‐1‐driven inflammation downstream of type I interferon. Science 2014; 345: 679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gold ES, Diercks AH, Podolsky I et al 25‐Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc Natl Acad Sci USA 2014; 111: 10666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koarai A, Yanagisawa S, Sugiura H et al 25‐Hydroxycholesterol enhances cytokine release and Toll‐like receptor 3 response in airway epithelial cells. Respir Res 2012; 13: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Umetani M, Ghosh P, Ishikawa T et al The cholesterol metabolite 27‐hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab 2014; 20: 172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature 2009; 460: 1122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B cells by the orphan G protein‐coupled receptor EBI2 shapes humoral immune responses. Immunity 2009; 31: 259–69. [DOI] [PubMed] [Google Scholar]
- 23. Liu C, Yang XV, Wu J et al Oxysterols direct B‐cell migration through EBI2. Nature 2011; 475: 519–23. [DOI] [PubMed] [Google Scholar]
- 24. Hannedouche S, Zhang J, Yi T et al Oxysterols direct immune cell migration via EBI2. Nature 2011; 475: 524–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yi T, Wang X, Kelly LM et al Oxysterol gradient generation by lymphoid stromal cells guides activated B cell movement during humoral responses. Immunity 2012; 37: 535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gatto D, Wood K, Caminschi I et al The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat Immunol 2013; 14: 446–53. [DOI] [PubMed] [Google Scholar]
- 27. Yi T, Cyster JG. EBI2‐mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. Elife 2013; 2: e00757.23682316 [Google Scholar]
- 28. Li J, Lu E, Yi T, Cyster JG. EBI2 augments Tfh cell fate by promoting interaction with IL‐2‐quenching dendritic cells. Nature 2016; 533: 110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu E, Dang EV, McDonald JG, Cyster JG. Distinct oxysterol requirements for positioning naive and activated dendritic cells in the spleen. Sci Immunol 2017; 2: eaal5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015; 517: 293–301. [DOI] [PubMed] [Google Scholar]
- 31. Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 2015; 348: aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Melo‐Gonzalez F, Hepworth MR. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology 2017; 150: 265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Randall TD, Mebius RE. The development and function of mucosal lymphoid tissues: a balancing act with micro‐organisms. Mucosal Immunol 2014; 7: 455–66. [DOI] [PubMed] [Google Scholar]
- 34. Buettner M, Lochner M. Development and function of secondary and tertiary lymphoid organs in the small intestine and the colon. Front Immunol 2016; 7: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carlsen HS, Baekkevold ES, Johansen FE, Haraldsen G, Brandtzaeg P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut 2002; 51: 364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moghaddami M, Cummins A, Mayrhofer G. Lymphocyte‐filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology 1998; 115: 1414–25. [DOI] [PubMed] [Google Scholar]
- 37. Pabst O, Herbrand H, Worbs T et al Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol 2005; 35: 98–107. [DOI] [PubMed] [Google Scholar]
- 38. Yeung MM, Melgar S, Baranov V et al Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR‐gammadelta expression. Gut 2000; 47: 215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lugering A, Ross M, Sieker M et al CCR6 identifies lymphoid tissue inducer cells within cryptopatches. Clin Exp Immunol 2010; 160: 440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nochi T, Denton PW, Wahl A, Garcia JV. Cryptopatches are essential for the development of human GALT. Cell Rep 2013; 3: 1874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lochner M, Ohnmacht C, Presley L et al Microbiota‐induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med 2011; 208: 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McNamee EN, Rivera‐Nieves J. Ectopic tertiary lymphoid tissue in inflammatory bowel disease: protective or provocateur? Front Immunol 2016; 7: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011; 474: 298–306. [DOI] [PubMed] [Google Scholar]
- 44. Surawicz CM, Belic L. Rectal biopsy helps to distinguish acute self‐limited colitis from idiopathic inflammatory bowel disease. Gastroenterology 1984; 86: 104–13. [PubMed] [Google Scholar]
- 45. Surawicz CM, Haggitt RC, Husseman M, McFarland LV. Mucosal biopsy diagnosis of colitis: acute self‐limited colitis and idiopathic inflammatory bowel disease. Gastroenterology 1994; 107: 755–63. [DOI] [PubMed] [Google Scholar]
- 46. Neurath M. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol 2017; 14: 688. [DOI] [PubMed] [Google Scholar]
- 47. Furtado GC, Pacer ME, Bongers G et al TNFalpha‐dependent development of lymphoid tissue in the absence of RORgammat(+) lymphoid tissue inducer cells. Mucosal Immunol 2014; 7: 602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lugering A, Kucharzik T, Soler D, Picarella D, Hudson JT III, Williams IR. Lymphoid precursors in intestinal cryptopatches express CCR6 and undergo dysregulated development in the absence of CCR6. J Immunol 2003; 171: 2208–15. [DOI] [PubMed] [Google Scholar]
- 49. Bouskra D, Brezillon C, Berard M et al Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008; 456: 507–10. [DOI] [PubMed] [Google Scholar]
- 50. McDonald KG, McDonough JS, Wang C, Kucharzik T, Williams IR, Newberry RD. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol 2007; 170: 1229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McDonald KG, McDonough JS, Dieckgraefe BK, Newberry RD. Dendritic cells produce CXCL13 and participate in the development of murine small intestine lymphoid tissues. Am J Pathol 2010; 176: 2367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baptista AP, Olivier BJ, Goverse G et al Colonic patch and colonic SILT development are independent and differentially regulated events. Mucosal Immunol 2013; 6: 511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Emgard J, Kammoun H, Garcia‐Cassani B et al Oxysterol sensing through the receptor GPR183 promotes the lymphoid‐tissue‐inducing function of innate lymphoid cells and colonic inflammation. Immunity 2018; 48: 120–32 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chu C, Moriyama S, Li Z et al Anti‐microbial functions of group 3 innate lymphoid cells in gut‐associated lymphoid tissues are regulated by g‐protein‐coupled receptor 183. Cell Rep 2018; 23: 3750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Satoh‐Takayama N, Serafini N, Verrier T et al The chemokine receptor CXCR6 controls the functional topography of interleukin‐22 producing intestinal innate lymphoid cells. Immunity 2014; 41: 776–88. [DOI] [PubMed] [Google Scholar]
- 56. Mackley EC, Houston S, Marriott CL et al CCR7‐dependent trafficking of RORgamma(+) ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun 2015; 6: 5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van de Pavert SA, Ferreira M, Domingues RG et al Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 2014; 508: 123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goverse G, Labao‐Almeida C, Ferreira M et al Vitamin A controls the presence of RORgamma+ innate lymphoid cells and lymphoid tissue in the small intestine. J Immunol 2016; 196: 5148–55. [DOI] [PubMed] [Google Scholar]
- 59. Kiss EA, Vonarbourg C, Kopfmann S et al Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011; 334: 1561–5. [DOI] [PubMed] [Google Scholar]
- 60. Lee JS, Cella M, McDonald KG et al AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol 2011; 13: 144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Uhlig HH, Powrie F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annu Rev Immunol 2018; 36: 755–81. [DOI] [PubMed] [Google Scholar]
- 62. Pearson C, Thornton EE, McKenzie B et al ILC3 GM‐CSF production and mobilisation orchestrate acute intestinal inflammation. Elife 2016; 5: e10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu J, McDuffie JE, Song J et al LC/MS/MS profiling of tissue oxysterols and its application in dextran sodium sulphate induced mouse colitis models. Curr Top Med Chem 2017; 17: 2781–90. [DOI] [PubMed] [Google Scholar]
- 64. Chalmin F, Rochemont V, Lippens C et al Oxysterols regulate encephalitogenic CD4(+) T cell trafficking during central nervous system autoimmunity. J Autoimmun 2015; 56: 45–55. [DOI] [PubMed] [Google Scholar]
- 65. Jakobsson T, Vedin LL, Hassan T et al The oxysterol receptor LXRbeta protects against DSS‐ and TNBS‐induced colitis in mice. Mucosal Immunol 2014; 7: 1416–28. [DOI] [PubMed] [Google Scholar]
- 66. Herold M, Breuer J, Hucke S et al Liver X receptor activation promotes differentiation of regulatory T cells. PLoS ONE 2017; 12: e0184985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cui G, Qin X, Wu L et al Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest 2011; 121: 658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Heinig M, Petretto E, Wallace C et al A trans‐acting locus regulates an anti‐viral expression network and type 1 diabetes risk. Nature 2010; 467: 460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jostins L, Ripke S, Weersma RK et al Host‐microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012; 491: 119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Andersen V, Christensen J, Ernst A et al Polymorphisms in NF‐kappaB, PXR, LXR, PPARgamma and risk of inflammatory bowel disease. World J Gastroenterol 2011; 17: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Molodecky NA, Soon IS, Rabi DM et al Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54 e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 72. Hou JK, Abraham B, El‐Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 2011; 106: 563–73. [DOI] [PubMed] [Google Scholar]
- 73. Mukherjee P, Hough G, Chattopadhyay A et al Transgenic tomatoes expressing the 6F peptide and ezetimibe prevent diet‐induced increases of IFN‐beta and cholesterol 25‐hydroxylase in jejunum. J Lipid Res 2017; 58: 1636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Progatzky F, Sangha NJ, Yoshida N et al Dietary cholesterol directly induces acute inflammasome‐dependent intestinal inflammation. Nat Commun 2014; 5: 5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rossin D, Calfapietra S, Sottero B, Poli G, Biasi F. HNE and cholesterol oxidation products in colorectal inflammation and carcinogenesis. Free Radic Biol Med 2017; 111: 186–95. [DOI] [PubMed] [Google Scholar]
- 76. Benned‐Jensen T, Smethurst C, Holst PJ et al Ligand modulation of the Epstein‐Barr virus‐induced seven‐transmembrane receptor EBI2: identification of a potent and efficacious inverse agonist. J Biol Chem 2011; 286: 29292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Benned‐Jensen T, Madsen CM, Arfelt KN et al Small molecule antagonism of oxysterol‐induced Epstein‐Barr virus induced gene 2 (EBI2) activation. FEBS Open Bio 2013; 3: 156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gessier F, Preuss I, Yin H et al Identification and characterization of small molecule modulators of the Epstein‐Barr virus‐induced gene 2 (EBI2) receptor. J Med Chem 2014; 57: 3358–68. [DOI] [PubMed] [Google Scholar]
- 79. Fessler MB. The challenges and promise of targeting the Liver X Receptors for treatment of inflammatory disease. Pharmacol Ther 2018; 181: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ungaro R, Chang HL, Cote‐Daigneault J, Mehandru S, Atreja A, Colombel JF. Statins associated with decreased risk of new onset inflammatory bowel disease. Am J Gastroenterol 2016; 111: 1416–23. [DOI] [PubMed] [Google Scholar]
- 81. Thapa D, Lee JS, Park SY et al Clotrimazole ameliorates intestinal inflammation and abnormal angiogenesis by inhibiting interleukin‐8 expression through a nuclear factor‐kappaB‐dependent manner. J Pharmacol Exp Ther 2008; 327: 353–64. [DOI] [PubMed] [Google Scholar]


