Figure 1.
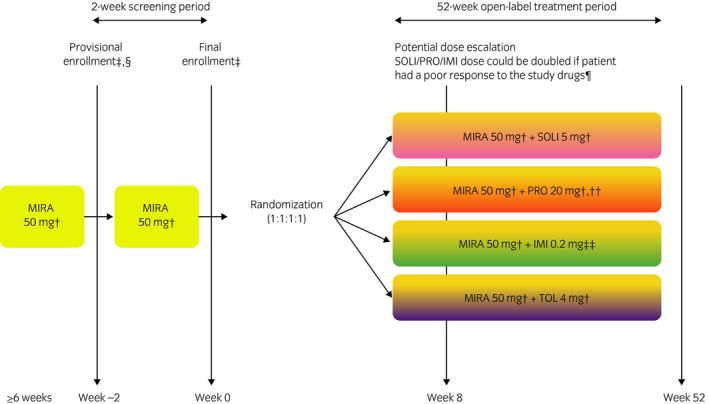
Study design. †Once daily. †Eligibility criteria were verified. §Informed consent was obtained. ¶Furthermore, the patient was considered by the investigator to have no safety concerns and agreed to the increased dose (in the event of a TEAE, the dose could be reduced to the initial dosage). ††If the PRO dose was doubled, patients received a 20‐mg dose twice daily. ††Twice daily (total daily dose shown).
