Summary
Background
Inhaled corticosteroids are effective for the treatment of equine asthma but they induce cortisol suppression with potential side effects.
Objectives
To study the efficacy of ciclesonide, an inhaled corticosteroid with an improved safety profile, on lung function, clinical signs related to airway obstruction, and serum cortisol levels in asthmatic horses exposed to a mouldy hay challenge.
Study design
Cross‐over placebo controlled, blinded, randomised experiment.
Methods
Sixteen horses were enrolled in three subsequent dose‐titration studies (8 horses/study) to investigate the effects of inhaled ciclesonide administered for 2 weeks at doses ranging from 450 to 2700 μg twice daily or 3712.5 μg once daily. Systemic dexamethasone (0.066 mg/kg per os) was our positive control. A placebo group was also studied. Lung function and clinical scores were blindly performed before and after 7 and 14 days of treatment. Serum cortisol was measured before and after 3, 5, 7, 10, 14 days of treatment as well as 3 and 7 days post treatment.
Results
After 7 days, dexamethasone induced a significant reduction in pulmonary resistance (from 2.5 ± 0.6 at day 0 to 1.1 ± 0.7 cm H2O/L/s), pulmonary elastance (5.0 ± 2.6 to 1.2 ± 1.0 cm H2O/L), and of the weighted clinical score (14.8 ± 4.7 to 8.0 ± 4.4). Similarly, ciclesonide 1687.5 μg twice daily significantly improved pulmonary resistance (2.7 ± 1.1 to 1.6 ± 0.8 cm H2O/L/s), pulmonary elastance (5.2 ± 3.1 to 2.2 ± 1.3 cm H2O/L), and weighted clinical score (13 ± 2.9 to 10.8 ± 4.2). Serum cortisol suppression (<50 nmol/L) systematically occurred with dexamethasone from day 3 of treatment up to day 3 post treatment, but not with ciclesonide at any tested doses. Placebo did not exert any significant beneficial effect.
Main limitations
Experimentally induced asthma exacerbations in horses might respond differently to treatment than naturally occurring exacerbations.
Conclusions
Inhaled ciclesonide is an effective treatment for horses with equine asthma. Serum cortisol was unaffected by treatment.
Keywords: horse, RAO, inhaled corticosteroids, lung function, inhalation therapy, cortisol
Introduction
Inhaled fluticasone at high doses is as effective as systemic corticosteroids at preventing exacerbations in severe asthmatic horses according to lung function parameters and signs of airway obstruction assessed with a clinical score 1. However, even high doses of inhaled fluticasone are not as effective as systemic dexamethasone for the treatment of equine asthma exacerbations 1. For this reason, systemically administered corticosteroids are indicated as the treatment of choice for equine asthma exacerbations in the most recent ACVIM Consensus Statement on equine asthma, because of their ability to rapidly improve clinical signs and lung function in affected horses 2. Inhaled corticosteroids have the advantage of being administered locally and at lower doses, potentially reducing side effects, making them an attractive alternative to systemic corticosteroid. Administration of inhaled fluticasone, beclomethasone and budesonide have been tested off‐label in asthmatic horses of all severities, with effective doses ranging from 2 mg once daily to 6 mg twice daily for fluticasone, 0.5–3.75 mg twice daily for beclomethasone, and 1.8 mg twice daily for budesonide 1, 3, 4, 5, 6, 7, 8. All of the above drugs reduce serum cortisol levels 1, 5, 7, 9, although this is neither associated with detectable alterations of the innate and adaptive immune response nor with the occurrence of severe adverse events 1, 10. Recently, short‐term low‐dose inhaled dexamethasone administration has been shown not to induce cortisol suppression in healthy horses 11, but its efficacy at improving the lung function of asthmatic horses remains unproven.
Ciclesonide is an inhaled corticosteroid with a low affinity for glucocorticoid receptors while in its inactive form. For this reason, it is considered an inhaled corticosteroid with an excellent safety profile. Ciclesonide enzymatic activation occurs within the lungs and increases the affinity of the drug for glucocorticoid receptors as much as 100–120 times 12. Due to its favourable safety profile, ciclesonide might be a valuable drug for asthma‐affected horses.
We hypothesised that: 1) inhaled ciclesonide effectively controls airway obstruction during a mouldy hay challenge in asthmatic horses, and that 2) its use does not reduce serum cortisol levels. Primarily, this study aims to investigate the effects of different doses of inhaled ciclesonide on the lung function and clinical signs related to airflow limitation in asthmatic horses in which controlled disease exacerbations were experimentally induced. The secondary objective is to assess the safety profile of ciclesonide in asthma‐affected horses, expressed in terms of serum cortisol levels, clinical adverse events, and alterations in haematology and blood biochemistry.
Materials and methods
Experimental design
Three crossover dose‐titration studies were performed in three consecutive years. They evaluated the effects of ciclesonide on the lung function, clinical signs, and selected blood parameters of asthmatic horses with experimentally induced controlled airway obstruction. The personnel performing clinical scores, lung function, data reporting, and statistical analysis were blinded to the treatment regimen of the horses. Study I was an explorative dose‐titration study aimed at determining the minimal treatment dose able to reverse airflow obstruction. Study II was designed to evaluate the effects of increased doses of ciclesonide. Inhalation devices with two different nozzles (A and B; a nozzle is the component of the device that regulate the duration of aerosol generation per actuation) were tested, to investigate whether reducing the duration of aerosol generation per actuation (more compliant with the increased breathing frequencies and decreased inspiratory time of asthmatic horses) could affect the outcomes of the study. Study III investigated the effect of once vs. twice daily administration of ciclesonide, using devices with the asthma‐adapted nozzle (B).
Eight horses participated in each study. Sample size calculation was extrapolated from previous reports 4, 13, 14 where 40–60% significant reduction in RL (our primary outcome) was induced with inhaled corticosteroids. The power of the two‐way analysis was set at 0.8, alpha was 0.05, and enrollment ratio was 1. The resulting sample size varied from two to seven horses per group. As we did not know the magnitude of the effect of ciclesonide, nor the variability in the horses' response to this treatment, eight horses were enrolled in each group. Personalised hay challenges able to induce a steady degree of airflow obstruction (ΔPL>15 cm H2O) in each animal were implemented for 2 weeks before the beginning of each study (conditioning) and then throughout the study period (Fig 1). Horses in each study were ranked by lung resistance (RL) measured during conditioning and divided within that ranking in pairs. Each pair was randomly assigned to group 1, 2, 3, or 4.
Figure 1.
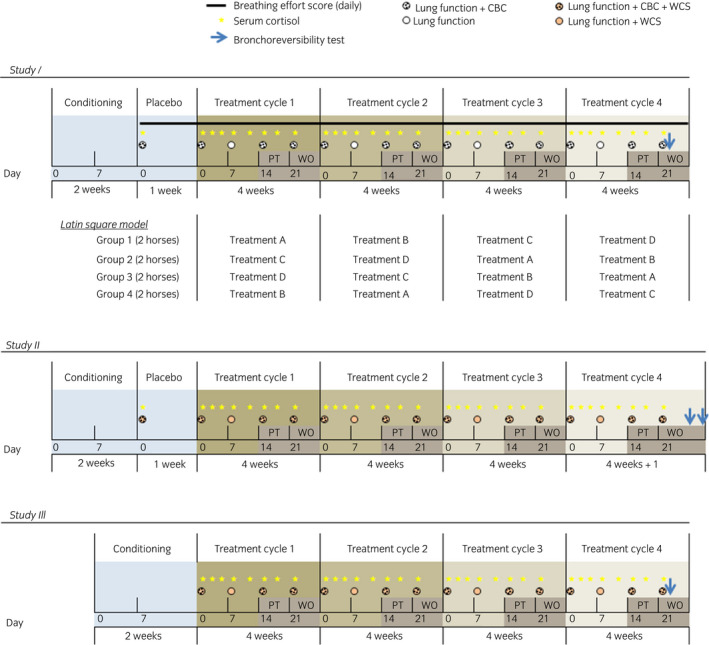
Study design. CBC, complete blood count; WCS, weighted clinical score; PT, post treatment week; WO, washout week. The Latin Square Model was applied to all studies.
In study I and study II, conditioning was followed by placebo (1 week), four cycles of treatment (each composed of 2 weeks of treatment, 1‐week post treatment, 1‐week washout), and bronchoreversibility tests. In study III, conditioning was immediately followed by the cycles of treatment; placebo was administered as one of the four treatments. Treatment randomisation during the treatment cycles was done respecting the Latin Square model, where all groups received all treatments at different times. Treatment order for the four groups was the same in all three studies (Fig 1). The severity of the respiratory condition was monitored using validated clinical scores 15, 16 by trained operators.
Clinical evaluation of the horses, lung function testing 17, bronchoreversibility testing, and blood sample collection were performed as reported in Figure 1. Methodological details are available in Supplementary Item 1. The procedures were performed in accordance with the guidelines of the Canadian Council on Animal Care. Animals developing life threatening signs (combination of laboured breathing, anorexia, or other indications of distress) had to be withdrawn from the study and treated by the attending clinician as directed by its condition. The study protocols were approved by the local Ethical Committee (Rech‐1490, Rech‐1548, Rech‐1594) and conform to the ARRIVE guidelines 18.
Treatments
Inhaled treatments were administered using an inhalation devicea employed in a previous study 7. It is composed of a nostril adapter, a transparent tube (chamber), and a soft mist inhaler core unit based on Respimat® technology (called device hereafter). All horses were trained to accept the device prior to the study. Actuations were performed at the beginning of inhalation to maximise drug delivery to the distal airways. The presence of a respiration indicator (“breath indicator”) located in the chamber wall of the nostril adapter allowed easy identification of the breathing phases of the horses by deflection.
Placebo was administered using the same device with cartridges containing the excipients (anhydrous ethanol, purified water, and hydrochloric acid) but lacking the active ingredient to be tested. Placebo and twice daily treatments were administered at approximately 08.00 and between 16.00 and 18.00. Systemic dexamethasone (0.066 mg/kg once daily in the morning) served as positive control and was administered orally mixed with molasses, using a plastic syringe.
In study I, the following treatments were administered using devices equipped with nozzle A: A) ciclesonide 450 μg (two actuations of 225 μg each) twice daily; B) ciclesonide 900 μg (four actuations) twice daily; C) ciclesonide 1800 μg (eight actuations) twice daily; D) dexamethasone.
In study II, the treatments tested were: A) ciclesonide 1687.5 μg (five actuations of 337.5 μg each) twice daily with device equipped with nozzle B; B) ciclesonide 2700 μg (eight actuations) twice daily with nozzle A; C) ciclesonide 2700 μg (eight actuations) twice daily with nozzle B; D) dexamethasone. The relevant difference between nozzle A and B was the drug release duration they induced (A: 1.5 s/actuation vs. B: 1.0 s/actuation).
In study III, the treatments tested were the following, all administered with devices equipped with nozzle B: A) placebo (0 μg ciclesonide, eight actuations) twice daily; B) ciclesonide 2700 μg (eight actuations of 337.5 μ each) twice daily; C) ciclesonide 3712.5 μg (11 actuations) once daily in the morning; D) ciclesonide 3712.5 μg (11 actuations) once daily in the evening.
Adverse events
Adverse events were defined as any changes in the horse health status requiring the attention of the attending veterinarian. Variation in the blood parameters tested were also recorded but not classified as AE when they occurred in the absence of any clinical sign.
Data analysis
Statistical analysis was performed with SAS software v.9.3b. In studies I and II, data obtained during the placebo phase were analysed separately using paired t test. Mixed linear models with horses as random factor were used to investigate the effect of treatment order and of the cycle of treatment (that is, whether initial values differed among treatments) on the parameters studied. A linear mixed model was also used to assess the effect of time, treatment, and their interaction in each study. A priori contrasts were performed to compare means of the same treatment at different time points and to compare means of different treatments at the same time point. Cochran‐Mantel‐Haenszel test was employed to analyse ordinal data (breathing effort score and weighted clinical score). The association between clinical scores and lung function parameters were evaluated using linear regression test. Paired t‐tests were employed to evaluate bronchoreversibility data. Additional details on statistical analysis are available in Supplementary Item 1.
Results
Horses
Details of the horses enrolled in each study are reported in Supplementary Item 1, together with data on the effect of treatment order and differences among cycles of treatment.
Study I
Placebo treatment did not alter significantly the breathing effort score, lung function parameters (ΔPL, RL, and pulmonary elastance, EL), serum cortisol levels, or CBC values (data not presented). Dexamethasone significantly decreased ΔPL, RL, and EL at day 7 and 14 of treatment. A return to baseline values was observed 7 days post treatment (day 21) (Fig 2a, b, and Supplementary Item 2a). Ciclesonide 450 and 900 μg did not significantly affect ΔPL, RL, and EL at any time points. Treatments with 1800 μg ciclesonide significantly decreased ΔPL and EL, but not RL, on days 7 and 14 of treatment but the effect was lost 7 days post treatment (day 21). At day 14, dexamethasone‐treated horses had significantly lower values of RL than horses treated with 450, 900, or 1800 μg ciclesonide and significantly lower values of EL than horses treated with 450 μg ciclesonide. Atropine administration significantly reduced ΔPL, RL, and EL in all horses, indicating the reversibility of the airway obstruction (Supplementary Item 3a–c). The 8‐point breathing effort score was not significantly affected by the treatments tested (Fig 2c).
Figure 2.
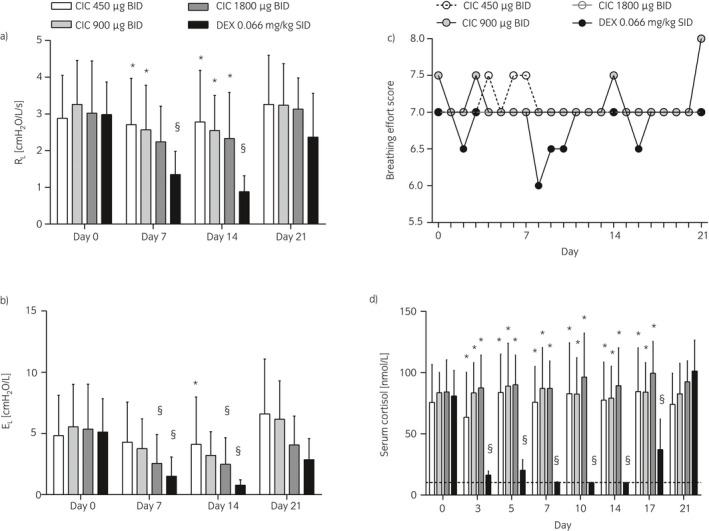
Study I. Lung function a)–b), breathing effort scores c), and serum cortisol d) of horses administered inhaled ciclesonide (CIC) at different doses or oral dexamethasone (DEX). Data are presented as means and s.d. a), b) and d) or medians c) across eight horses at each day. RL, respiratory resistance; EL, respiratory elastance. §Different from day 0 for the same treatment (P<0.001); *Different from dexamethasone at the same time point (P≤0.003).
Treatments with 450, 900, and 1800 μg of ciclesonide did not alter significantly serum cortisol over time (days 3, 5, 7, 10, 14, 17, and 21 compared to day 0). During ciclesonide treatment, four horses accounted for 12 (out of 64) cortisol measures below normal ranges (<50 nmol/L). Dexamethasone significantly decreased serum cortisol on days 3, 5, 7, 10, 14, (treatment) and 17 (3 days post treatment) compared to day 0. Cortisol had returned to baseline on day 21 (7 days post treatment). On days 3 to 17, serum cortisol levels were significantly lower in dexamethasone‐treated than in ciclesonide‐treated horses (Fig 2d). Ciclesonide did not alter significantly packed cell volume (PCV), total protein, WBC counts, segmented neutrophils, or lymphocyte values. Dexamethasone significantly decreased blood lymphocytes on day 14 and total proteins on day 21 compared to day 0 (Table 1).
Table 1.
Haematology and blood biochemistry findings in study I
| Ciclesonide 450 μg | Ciclesonide 900 μg | Ciclesonide 1800 μg | Dexamethasone | |
|---|---|---|---|---|
| PCV [%] | ||||
| Day 0 | 36 ± 4 | 37 ± 5 | 36 ± 7 | 36 ± 7 |
| Day 14 | 35 ± 4 | 34 ± 6 | 34 ± 6 | 37 ± 5 |
| Day 21 | 37 ± 5 | 36 ± 5 | 35 ± 6 | 35 ± 5 |
| Total proteins [g/L] | ||||
| Day 0 | 75.1 ± 7.3 | 75.6 ± 6.9 | 72.6 ± 9.0 | 74.9 ± 7.4 |
| Day 14 | 73.9 ± 6.1 | 74.4 ± 4.7 | 71.9 ± 6.4 | 72.4 ± 2.3 |
| Day 21 | 75.2 ± 7.1b | 75.1 ± 6.6b | 72.0 ± 08.9 | 65.7 ± 2.8a |
| WBC [×109/L] | ||||
| Day 0 | 7.1 ± 1.6 | 7.5 ± 1.1 | 7.1 ± 1.6 | 7.3 ± 1.5 |
| Day 14 | 7.3 ± 2.0 | 6.9 ± 1.4 | 7.8 ± 1.6 | 8.3 ± 1.7 |
| Day 21 | 8.1 ± 1.8 | 7.3 ± 2.8 | 7.4 ± 1.0 | 6.5 ± 1.6 |
| Segmented neutrophils [×109/L] | ||||
| Day 0 | 4.7 ± 1.3 | 5.2 ± 1.1 | 4.6 ± 1.3 | 4.8 ± 1.3 |
| Day 14 | 5.0 ± 1.7 | 4.4 ± 1.1 | 5.4 ± 1.7 | 6.2 ± 1.3 |
| Day 21 | 5.5 ± 1.5 | 4.9 ± 2.5 | 9.4 ± 1.1 | 4.3 ± 1.2 |
| Lymphocytes [×109/L] | ||||
| Day 0 | 2.0 ± 0.4 | 1.8 ± 0.3 | 1.9 ± 0.5 | 2.1 ± 0.7 |
| Day 14 | 2.0 ± 0.4 | 2.0 ± 0.5 | 2.0 ± 0.6 | 1.7 ± 0.5a |
| Day 21 | 2.2 ± 0.5b | 1.9 ± 0.3 | 2.1 ± 0.6 | 1.8 ± 0.4 |
Data presented as mean ± s.d. PCV, packed cell volume; WBC, white blood cells.
Different from the same treatment at day 0 (P≤0.002).
Different from dexamethasone at the same time point (P≤0.007).
Adverse events observed in study I were most frequently represented by ringworm lesions, observed in 7/8 horses with onset during the washout week of the first cycle of treatment. No other AE was observed in >1 horse during ciclesonide treatment. A detailed description of the AE is available online.
Study II
Placebo treatment did not alter significantly any of the parameters studied (data not shown). All treatments studied induced a statistically significant decrease in ΔPL, RL, and EL at day 7 and day 14 compared to day 0. The only exceptions were ΔPL of horses treated with 1687.5 μg of ciclesonide at day 7 and EL of horses treated with 2700 μg of ciclesonide (nozzle B) at day 7 (Fig 3a, b, and Supplementary Item 2b). No significant differences were observed at any time points among the treatments tested. The WCS significantly decreased with all treatments at day 14. The WCS of horses treated with dexamethasone and with 2700 μg ciclesonide (nozzle B) were significantly reduced also at day 7 and day 21 compared to day 0 (Fig 3c). Overall, the 23‐point WCS employed predicted ΔPL, RL, and EL with r2 values of 0.48, 0.38, and 0.47, respectively. The results of bronchoreversibility tests performed at the end of study II have been published previously 19 and showed that all horses responded to the bronchodilator.
Figure 3.
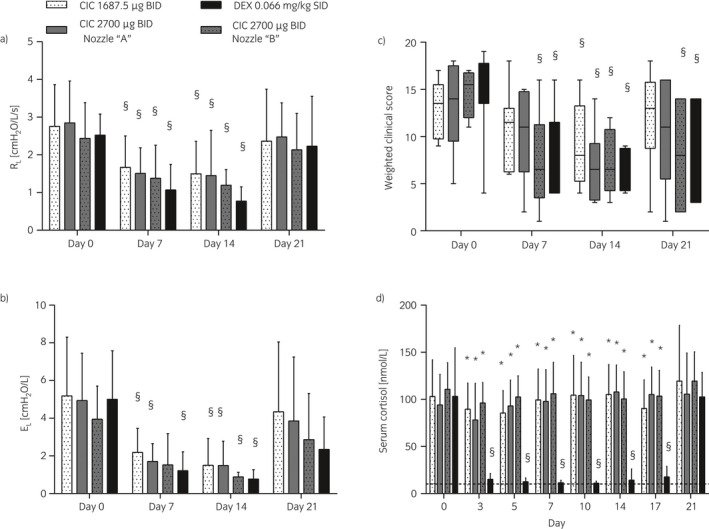
Study II. Lung function a), b), weighted clinical scores c), and serum cortisol d) of horses administered oral dexamethasone (DEX) or inhaled ciclesonide (CIC) at different doses and/or devices a) vs. b). Data are presented as means and s.d. a), b), d) or interquartile ranges and mix‐to‐max values c) across eight horses at each day. RL, respiratory resistance; EL, respiratory elastance. §Different from day 0 for the same treatment (P≤0.003); *Different from dexamethasone at the same time point (P<0.0001).
Serum cortisol was significantly lower on day 0 of the first vs. second (P = 0.01) and third (P = 0.048) cycles of treatment. Only dexamethasone reduced significantly serum cortisol on days 3 to 17 compared to day 0. On these days, cortisol values in dexamethasone‐treated horses were significantly lower compared to those of horses receiving other treatments (Fig 3d). During ciclesonide treatment, one horse accounted for six (out of 64) cortisol measures below normal ranges: one during treatment A (day 5) and five during B (days 0–10). Overall, PCV and total proteins were significantly lower in all horses on day 0 of the fourth cycle of treatment compared to the first (P = 0.006 and P = 0.02, respectively) and second (P = 0.02 and P = 0.03) ones. Similarly, WBC was lower on day 0 of the fourth than on the first cycle of treatment (P = 0.02). All values remained within normal ranges, however. Dexamethasone significantly decreased PCV on day 14 and total proteins on days 14 and 21 compared to day 0. On day 21, total proteins were significantly lower in dexamethasone‐treated horses compared to those treated with 1687.5 and 2700 μg (device with nozzle A) of ciclesonide. Ciclesonide did not alter significantly any of the blood parameters studied (Table 2).
Table 2.
Haematology and blood biochemistry findings in study II
| Ciclesonide 1687.5 μg | Ciclesonide 2700 μg (A) | Ciclesonide 2700 μg (B) | Dexamethasone | |
|---|---|---|---|---|
| PCV [%] | ||||
| Day 0 | 34 ± 4 | 35 ± 5 | 34 ± 3 | 36 ± 4 |
| Day 14 | 32 ± 5 | 32 ± 4 | 32 ± 4 | 31 ± 4a |
| Day 21 | 34 ± 4 | 33 ± 5 | 34 ± 4 | 35 ± 4 |
| Total proteins [g/L] | ||||
| Day 0 | 71.8 ± 6.4 | 71.6 ± 8.0 | 70.6 ± 3.7 | 75.1 ± 6.3 |
| Day 14 | 70.8 ± 5.1 | 70.1 ± 4.1 | 69.3 ± 4.9 | 69.8 ± 3.6a |
| Day 21 | 73.0 ± 4.5b | 71.5 ± 5.8b | 70.4 ± 6.3 | 65.5 ± 3.8a |
| WBC [×109/L] | ||||
| Day 0 | 6.8 ± 2.2 | 7.4 ± 2.0 | 6.49 ± 1.4 | 7.4 ± 1.9 |
| Day 14 | 7.6 ± 2.8 | 7.5 ± 1.7 | 7.0 ± 2.0 | 8.3 ± 1.8 |
| Day 21 | 7.1 ± 2.2 | 6.8 ± 2.2 | 7.2 ± 2.0 | 7.0 ± 1.1 |
| Segmented neutrophils [×109/L] | ||||
| Day 0 | 4.5 ± 1.6 | 4.9 ± 1.4 | 4.2 ± 1.2 | 4.8 ± 1.5 |
| Day 14 | 5.2 ± 2.5 | 4.8 ± 1.1 | 4.7 ± 2.0 | 6.2 ± 1.4 |
| Day 21 | 4.5 ± 1.8 | 4.4 ± 1.7 | 4.8 ± 1.5 | 4.8 ± 1.0 |
| Lymphocytes [×109/L] | ||||
| Day 0 | 1.9 ± 0.5 | 2.0 ± 0.6 | 1.8 ± 0.4 | 2.1 ± 0.6 |
| Day 14 | 1.9 ± 0.3 | 2.1 ± 0.4 | 1.7 ± 0.4 | 1.7 ± 0.6 |
| Day 21 | 2.0 ± 0.4 | 2.0 ± 0.4 | 2.0 ± 0.6 | 1.7 ± 0.3 |
Data presented as mean ± s.d. PCV, packed cell volume; WBC, white blood cells.
Different from the same treatment at day 0 (P≤0.002).
Different from dexamethasone at the same time point (P≤0.002).
The most commonly observed AE was ocular irritation and discharge (six episodes in five horses, of which five during ciclesonide treatment and one during post ciclesonide treatment week). More details are available online.
Study III
ΔPL, RL, EL, and weighted clinical score significantly decreased on day 7 and 14 compared to day 0 only with 2700 μg ciclesonide twice daily. A significant reduction in RL was also observed after 14 days of treatment with 3712.5 μg ciclesonide administered once in the evening. On day 14, values of ΔPL, RL, EL, and weighted clinical score of all ciclesonide‐treated horses were significantly lower compared to those observed in the placebo‐treated animals. (Fig 4a, b, c, and Supplementary Item 2c). At the end of the study period, N‐butyl‐scopolamine administration significantly reduced ΔPL, RL, and EL in all horses (Supplementary Item 3d–f). When horses were arbitrarily classified as having mild, moderate, and severe obstruction based on RL (1.0–1.8, 1.8–2.5, and >2.5 cm H2O/L/s, respectively) and not accounting for repeated measures, a score ≥15 identified horses with severe obstruction with 70% sensitivity and 62% specificity. Horses with moderate‐to‐severe airway obstruction were correctly identified with a score ≥11 with 82% sensitivity and 70% specificity. The weighted clinical score employed is a good predictor of RL during disease exacerbations (Fig 5).
Figure 4.
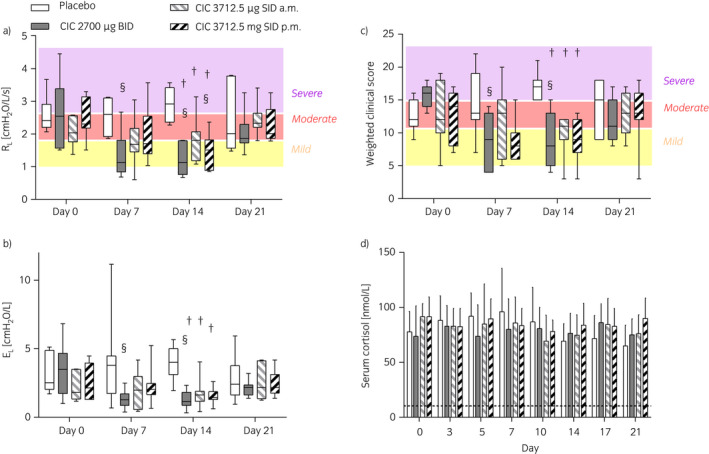
Study III, Lung function a), b), weighted clinical score c), and serum cortisol values d) of horses administered inhaled placebo or ciclesonide (CIC) at different doses and frequency. In a) and c), horses were subjectively classified as having severe, moderate, or mild airway obstruction based on RL and weighted clinical score. Data are presented as interquartile ranges and mix‐to‐max values in a)–c) and as means and s.d. d) across eight horses at each day. EL, respiratory elastance. §Different from day 0 for the same treatment (P≤0.001); †Different from placebo at the same time point (P≤0.0004).
Figure 5.
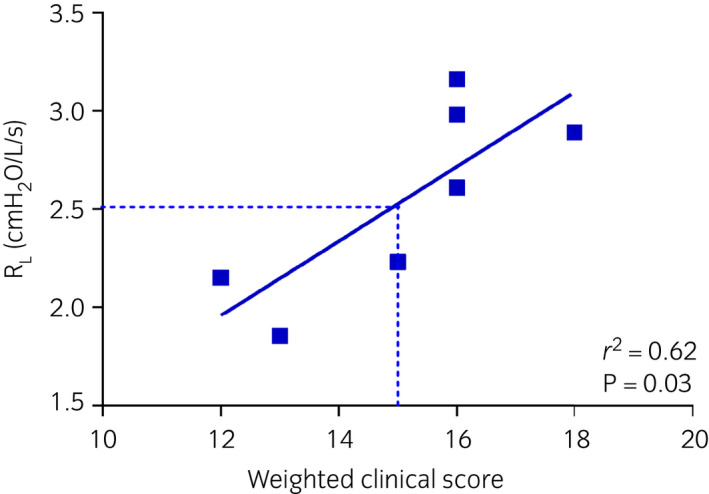
Study III. Relationship between respiratory resistance (RL) and weighted clinical score during equine asthma exacerbations. Each point represents the mean (RL) or median (weighted clinical score) of repeated observations on the same horse in the absence of treatment (placebo days 0–7–14–21, ciclesonide treatment days 0).
None of the treatments tested significantly decreased serum cortisol (Fig 4d). During ciclesonide treatment, four horses accounted for seven (out of 56) cortisol measures below the normal level (three horses during treatment B, on days 0, 5, 7; and two horses during C, on days 5–7–10 and 14). During placebo, two horses accounted for four cortisol measures below the normal level (on days 7 and 10–17–21). Treatment did not significantly affect plasma protein concentration, PCV, WBC, neutrophils, and lymphocytes counts (Table 3).
Table 3.
Haematology and blood biochemistry findings in study III
| Placebo | Ciclesonide 2700 μg | Ciclesonide 3712.5 μg am | Ciclesonide 3712.5 μg pm | |
|---|---|---|---|---|
| PCV [%] | ||||
| Day 0 | 34 ± 5 | 35 ± 4 | 35 ± 2 | 35 ± 4 |
| Day 14 | 34 ± 5 | 34 ± 3 | 34 ± 4 | 34 ± 5 |
| Day 21 | 32 ± 4 | 33 ± 3 | 36 ± 4 | 33 ± 5 |
| Total proteins [g/L] | ||||
| Day 0 | 68.2 ± 2.4 | 69.7 ± 3.1 | 71.1 ± 3.6 | 69.9 ± 3.7 |
| Day 14 | 71.1 ± 2.3 | 69.7 ± 2.8 | 69.4 ± 3.9 | 68.0 ± 4.2 |
| Day 21 | 70.0 ± 2.0 | 67.1 ± 3.4 | 69.0 ± 4.2 | 68.0 ± 3.6 |
| WBC [×109/L] | ||||
| Day 0 | 6.7 ± 0.8 | 6.8 ± 0.6 | 6.9 ± 1.0 | 6.6 ± 1.3 |
| Day 14 | 7.0 ± 1.5 | 7.4 ± 1.6 | 7.3 ± 1.2 | 7.2 ± 1.1 |
| Day 21 | 6.7 ± 1.3 | 6.3 ± 1.0 | 6.8 ± 1.3 | 6.3 ± 0.8 |
| Segmented neutrophils [×109/L] | ||||
| Day 0 | 4.1 ± 1.0 | 4.0 ± 0.8 | 4.4 ± 1.0 | 3.7 ± 1.2 |
| Day 14 | 4.3 ± 1.3 | 4.6 ± 1.4 | 4.4 ± 1.0 | 4.4 ± 1.2 |
| Day 21 | 3.9 ± 1.1 | 3.5 ± 0.8 | 3.7 ± 1.1 | 3.4 ± 0.7 |
| Lymphocytes [×109/L] | ||||
| Day 0 | 2.3 ± 0.5 | 2.5 ± 0.6 | 2.2 ± 0.3 | 2.5 ± 0.4 |
| Day 14 | 2.3 ± 0.2 | 2.5 ± 0.6 | 2.5 ± 0.5 | 2.4 ± 0.3 |
| Day 21 | 2.5 ± 0.3 | 2.5 ± 0.5 | 2.7 ± 0.5 | 2.5 ± 0.3 |
Data presented as mean ± s.d. PCV, packed cell volume; WBC, white blood cells.
Adverse events consisted mainly of epiphora and pruritus. Except for epiphora, that was observed in five horses during ciclesonide treatments (six observations) and in two horses (two observations) during placebo/post treatment/washout, the AE were equally observed during ciclesonide treatments or placebo/post treatment/washout weeks. Additional details are provided online.
Three out of seven horses achieved RL normalisation (RL<1 cm H2O/L/s) after 14 days of 2700 μg ciclesonide twice daily. Only 2/7 horses reached RL normalisation with 3712.5 μg ciclesonide once in the evening, and 0/7 when the same dose was administered in the morning. The number of horses achieving RL normalisation 20 min after N‐butyl‐scopolamine‐induced bronchodilation was 2/7.
Discussion
Inhaled corticosteroids improve the lung function during experimentally induced exacerbations in asthmatic horses 1, 3, 4, 6, 7. However, their use in horses is off‐label and all formerly used inhaled corticosteroids significantly decrease serum cortisol 1, 9. Having inhaled corticosteroids for use in horses with an improved safety profile would be suitable for prevention and treatment of equine asthma and related conditions 1. The present studies investigated for the first time the effects of increased doses of inhaled ciclesonide on lung function, clinical signs related to airflow limitation, and serum cortisol levels in asthmatic horses. The first experiment was designed as a dose‐titration study; we then indirectly investigated the effects of a shortened drug delivery time (asthma‐adapted nozzle, study II) and of different administration frequencies/time (study III). Our results indicate that ciclesonide reverses airway obstruction in horses with asthma, with a favourable safety profile. Indeed, serum cortisol was not significantly affected by ciclesonide administration at any of the doses tested.
Ciclesonide is a pro‐drug, enzymatically hydrolysed to a pharmacologically active metabolite desisobutyryl‐ciclesonide (des‐CIC) following inhalative application by carboxylesterases present within the bronchial epithelial cells. Des‐CIC has anti‐inflammatory activity with affinity for the glucocorticoid receptor that is 100–120 times higher than the parent compound. The anti‐inflammatory effects of des‐CIC in human asthma are thought to be primarily mediated via inhibition of tumour necrosis factor‐alpha and the Th‐2 cytokine interleukin‐4 in airway epithelial cells 12, two cytokines implicated in the pathogenesis of equine asthma 20, 21. The maximal dose of ciclesonide to be tested in the first study was based on the clinical equivalence observed in human patients between fluticasone propionate and ciclesonide (80 μg of ciclesonide are considered clinically equivalent to 100 μg of fluticasone 22) and on previous experiments performed in horses during which fluticasone propionate was shown to be effective at doses ≥2000 μg/day 1, 3, 4. The decision to test lower doses initially was made to determine the minimal effective dose for treating asthmatic horses. Study III suggests that administration of 2700 μg ciclesonide twice daily might be the most effective dose of the treatment, with significant RL reduction already at day 7 compared to day 0, and with no differences noticed with the other tested doses. Although ciclesonide induced significant amelioration of the lung function, especially at the highest doses tested, RL normalisation was not reached in all subjects. The same was observed with dexamethasone, most likely because inhibition of some of the factors causing obstruction (i.e. mucus hypersecretion, bronchial remodelling) may take >14 days.
Ciclesonide is characterised by low oral bioavailability (<1%) and low systemic exposure. It displays high plasma protein binding (~99% vs. 71–98% of most ICSs) and rapid systemic clearance. As systemic side effects of inhaled corticosteroids result from the interaction of unbound systemically available fraction of inhaled corticosteroids with glucocorticoid receptors expressed by non‐pulmonary cells, ciclesonide has an excellent safety profile 12. Suppression of endogenous cortisol levels following inhaled corticosteroid therapy is indicative of an effect on the hypothalamic–pituitary–adrenal axis and represents undesired systemic activity. Prolonged ciclesonide treatment did not suppress serum cortisol levels in our study. Cortisol levels below normal ranges were occasionally detected in placebo and ciclesonide‐treated animals. Whether this was due to cumulative cortisol suppression 23, to circadian hormonal variation, or to other causes remains undetermined.
Significant reduction in RL on day 7 was observed with ciclesonide doses ≥2700 and ≥1687.5 μg twice daily when nozzle A and B were used, respectively. Similar outcomes were observed for EL, suggesting an effective deposition of ciclesonide throughout the bronchial tree with both nozzles, but a possibly improved performance of nozzle B, despite no significant differences were noted when nozzles were directly compared at the same drug dosage. The effects of ciclesonide 2700 μg twice daily on RL and weighted clinical score were observed on day 7 in horses with mild‐to‐severe airflow obstruction, with ≥30% improvement even in horses showing mild airflow limitation. Ciclesonide 3712.5 μg once daily significantly improved RL only when delivered in the evening. A significant effect of dosage timing on drug efficacy is reported for other inhaled corticosteroids, with evening administration showing better results 24. Ciclesonide effect on lung function was lost 1‐week after discontinuation of the therapy. Contrarily, weighted clinical score improvement remained significant up to 1‐week post treatment in horses receiving ciclesonide 2700 μg twice daily and dexamethasone in study II. This discrepancy could be due to the exacerbation‐related development/worsening of air trapping in the horses' lungs, which might prevent the transmission of lung sounds during auscultation, falsely reducing the scores in this phase. Alternatively, the score parameters not directly related to lung resistance (e.g. nasal discharge, coughing, and tracheal sounds) might have introduced a bias. Overall, however, the 23‐point weighted clinical score allowed the identification of horses experiencing airway obstruction in most cases and was able to predict the degree of airflow obstruction (mild/moderate vs. severe) with 82% sensitivity and 70% specificity. These findings indicate that the clinical score employed can be applied clinically and can serve as a practical tool for the practitioners to monitor airway dysfunction.
Ciclesonide was administered using a device composed of a soft mist inhaler core based on Respimat® technology, and an adaptor for the horse nostril comprising a respiration indicator in the chamber wall, which facilitates the identification of the breathing phases of the horse by deflection. The soft mist inhaler forces a metered dose of the drug solution through a nozzle, producing two fine jets of liquid that converge at a preset angle generating the soft mist. The advantages of the soft mist inhaler are: the high fine particle fraction (~65–80% particles <5 μm; this fraction can reach the small airways and alveoli), the slow velocity of ejection of the soft mist, and the consequent relatively long generation time of the aerosol cloud. Altogether, these factors result in larger amounts of the drug delivered to the lungs 25. In this study, two different nozzles were tested with slight differences in clinical outcomes. It was noticed that when the horses' respiratory rate exceeded 30 bpm, the inspiratory time was too short for the delivery time of the inhaler. However, thanks to the inhaler design, most of the released ciclesonide remained available in the chamber, for inhalation during subsequent breathing cycles.
In summary, 1‐week treatment with inhaled ciclesonide significantly improved lung function and clinical signs of horses with equine asthma in exacerbation, without suppressing serum cortisol. Overall, the effect on lung function and clinical signs of airway obstruction remain constant from day 7 to day 14 of treatment. Maximal clinical effects are observed with 2700 μg of ciclesonide administered twice daily in horses with experimentally induced equine asthma exacerbations.
Authors' declaration of interests
J.P. Lavoie and M. Bullone have a consultancy agreement with the company that financed the study. B. Albrecht and M. von Salis‐Soglio are employed by the company that financed the study.
Ethical animal research
The study protocols were approved by the Animal Care and Use Committee of the Université de Montréal (Rech‐1490, Rech‐1548, Rech‐1594).
Owner informed consent
Not applicable.
Sources of funding
Support for this study was provided by Boehringer Ingelheim Vetmedica GmbH.
Authorship
J.‐P. Lavoie, B. Albrecht, and M. von Salis‐Soglio contributed to study design, data analysis, interpretation, and preparation of the manuscript. M. Bullone, N. Rodrigues, and P. Germim participated in data collection, analysis and interpretation, preparation of the manuscript. All authors have read and approved the final version of the manuscript.
Supporting information
Supplementary Item 1: Methodological details.
Supplementary Item 2: Transpulmonary pressure.
Supplementary Item 3: Bronchoreversibility tests.
Acknowledgements
The authors thank Gaëlle Hirsch, Mylène Chevigny, Ruby Yoana Murcia Robayo, Catheryna Ouimet, Alexandra Walling, Amandine Vargas, and Maude de Lagarde for technical help throughout the study, and Guy Beauchamp for statistical analyses.
Manufacturers' addresses
Boehringer Ingelheim Vetmedica GmbH, Ingelheim am Rhein, Germany.
SAS Institute Inc., Cary, North Carolina, USA.
References
- 1. Robinson, N.E. , Berney, C. , Behan, A. and Derksen, F.J. (2009) Fluticasone propionate aerosol is more effective for prevention than treatment of recurrent airway obstruction. J. Vet. Intern. Med. 23, 1247‐1253. [DOI] [PubMed] [Google Scholar]
- 2. Couetil, L.L. , Cardwell, J.M. , Gerber, V. , Lavoie, J.P. , Leguillette, R. and Richard, E.A. (2016) Inflammatory airway disease of horses‐revised consensus statement. J. Vet. Intern. Med. 30, 503‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bullone, M. , Vargas, A. , Elce, Y. , Martin, J.G. and Lavoie, J.P. (2017) Fluticasone/salmeterol reduces remodelling and neutrophilic inflammation in severe equine asthma. Sci. Rep. 7, 8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leclere, M. , Lavoie‐Lamoureux, A. , Joubert, P. , Relave, F. , Lanctot Setlakwe, E. , Beauchamp, G. , Couture, C. , Martin, J.G. and Lavoie, J.P. (2012) Corticosteroids and antigen avoidance decrease airway smooth muscle mass in an equine asthma model. Am. J. Respir. Cell Mol. Biol. 47, 589‐596. [DOI] [PubMed] [Google Scholar]
- 5. Rush, B.R. , Raub, E.S. , Thomsen, M.M. , Davis, E.G. , Matson, C.J. and Hakala, J.E. (2000) Pulmonary function and adrenal gland suppression with incremental doses of aerosolized beclomethasone dipropionate in horses with recurrent airway obstruction. J. Am. Vet. Med. Assoc. 217, 359‐364. [DOI] [PubMed] [Google Scholar]
- 6. Leguillette, R. , Tohver, T. , Bond, S.L. , Nicol, J.A. and McDonald, K.J. (2017) Effect of dexamethasone and fluticasone on airway hyperresponsiveness in horses with inflammatory airway disease. J. Vet. Intern. Med. 31, 1193‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lavoie, J.P. , Leclere, M. , Rodrigues, N. , Lemos, K.R. , Bourzac, C. , Lefebvre‐Lavoie, J. , Beauchamp, G. and Albrecht, B. (2019) Efficacy of inhaled budesonide for the treatment of severe equine asthma. Equine Vet. J. 51, 401‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ammann, V.J. , Vrins, A.A. and Lavoie, J.P. (1998) Effects of inhaled beclomethasone dipropionate on respiratory function in horses with chronic obstructive pulmonary disease (COPD). Equine Vet. J. 30, 152‐157. [DOI] [PubMed] [Google Scholar]
- 9. Munoz, T. , Leclere, M. , Jean, D. and Lavoie, J.P. (2015) Serum cortisol concentration in horses with heaves treated with fluticasone proprionate over a 1 year period. Res. Vet. Sci. 98, 112‐114. [DOI] [PubMed] [Google Scholar]
- 10. Dauvillier, J. , Felippe, M.J. , Lunn, D.P. , Lavoie‐Lamoureux, A. , Leclere, M. , Beauchamp, G. and Lavoie, J.P. (2011) Effect of long‐term fluticasone treatment on immune function in horses with heaves. J. Vet. Intern. Med. 25, 549‐557. [DOI] [PubMed] [Google Scholar]
- 11. Haspel, A.D. , Giguere, S. , Hart, K.A. , Berghaus, L.J. and Davis, J.L. (2018) Bioavailability and tolerability of nebulised dexamethasone sodium phosphate in adult horses. Equine Vet. J. 50, 85‐90. [DOI] [PubMed] [Google Scholar]
- 12. Mukker, J.K. , Singh, R.S. and Derendorf, H. (2016) Ciclesonide: a pro‐soft drug approach for mitigation of side effects of inhaled corticosteroids. J. Pharm. Sci. 105, 2509‐2514. [DOI] [PubMed] [Google Scholar]
- 13. Cornelisse, C.J. , Robinson, N.E. , Berney, C.E. , Kobe, C.A. , Boruta, D.T. and Derksen, F.J. (2004) Efficacy of oral and intravenous dexamethasone in horses with recurrent airway obstruction. Equine Vet. J. 36, 426‐430. [DOI] [PubMed] [Google Scholar]
- 14. Lavoie, J.P. , Leguillette, R. , Pasloske, K. , Charette, L. , Sawyer, N. , Guay, D. , Murphy, T. and Hickey, G.J. (2002) Comparison of effects of dexamethasone and the leukotriene D4 receptor antagonist L‐708,738 on lung function and airway cytologic findings in horses with recurrent airway obstruction. Am. J. Vet. Res. 63, 579‐585. [DOI] [PubMed] [Google Scholar]
- 15. Robinson, N.E. , Olszewski, M.A. , Boehler, D. , Berney, C. , Hakala, J. , Matson, C. and Derksen, F.J. (2000) Relationship between clinical signs and lung function in horses with recurrent airway obstruction (heaves) during a bronchodilator trial. Equine Vet. J. 32, 393‐400. [DOI] [PubMed] [Google Scholar]
- 16. Tesarowski, D.B. , Viel, L. and McDonell, W.N. (1996) Pulmonary function measurements during repeated environmental challenge of horses with recurrent airway obstruction (heaves). Am. J. Vet. Res. 57, 1214‐1219. [PubMed] [Google Scholar]
- 17. Jean, D. , Vrins, A. and Lavoie, J.P. (1999) Monthly, daily, and circadian variations of measurements of pulmonary mechanics in horses with chronic obstructive pulmonary disease. Am. J. Vet. Res. 60, 1341‐1346. [PubMed] [Google Scholar]
- 18. Kilkenny, C. , Browne, W.J. , Cuthill, I.C. , Emerson, M. and Altman, D.G. (2012) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage 20, 256‐260. [DOI] [PubMed] [Google Scholar]
- 19. de Lagarde, M. , Rodrigues, N. , Chevigny, M. , Beauchamp, G. , Albrecht, B. and Lavoie, J.P. (2014) N‐butylscopolammonium bromide causes fewer side effects than atropine when assessing bronchoconstriction reversibility in horses with heaves. Equine Vet. J. 46, 474‐478. [DOI] [PubMed] [Google Scholar]
- 20. Laan, T.T. , Bull, S. , Pirie, R. and Fink‐Gremmels, J. (2006) The role of alveolar macrophages in the pathogenesis of recurrent airway obstruction in horses. J. Vet. Intern. Med. 20, 167‐174. [DOI] [PubMed] [Google Scholar]
- 21. Lavoie, J.P. , Maghni, K. , Desnoyers, M. , Taha, R. , Martin, J.G. and Hamid, Q.A. (2001) Neutrophilic airway inflammation in horses with heaves is characterized by a Th2‐type cytokine profile. Am. J. Respir. Crit. Care Med. 164, 1410‐1413. [DOI] [PubMed] [Google Scholar]
- 22. GINA Guidelines . (2018) Global Strategy for Asthma Management and Prevention.
- 23. Meibohm, B. , Hochhaus, G. , Rohatagi, S. , Mollmann, H. , Barth, J. , Wagner, M. , Krieg, M. , Stockmann, R. and Derendorf, H. (1997) Dependency of cortisol suppression on the administration time of inhaled corticosteroids. J. Clin. Pharmacol. 37, 704‐710. [DOI] [PubMed] [Google Scholar]
- 24. Noonan, M. , Karpel, J.P. , Bensch, G.W. , Ramsdell, J.W. , Webb, D.R. , Nolop, K.B. and Lutsky, B.N. (2001) Comparison of once‐daily to twice‐daily treatment with mometasone furoate dry powder inhaler. Ann. Allergy Asthma Immunol. 86, 36‐43. [DOI] [PubMed] [Google Scholar]
- 25. Dalby, R. , Spallek, M. and Voshaar, T. (2004) A review of the development of Respimat Soft Mist Inhaler. Int. J. Pharm. 283, 1‐9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Item 1: Methodological details.
Supplementary Item 2: Transpulmonary pressure.
Supplementary Item 3: Bronchoreversibility tests.


