Abstract
Introduction
Patients with bleeding disorders may experience limitations in sports participation and physical activity. Several studies on sports participation have been performed in haemophilia patients, but studies in patients with von Willebrand disease (VWD) are lacking.
Aim
We assessed the sports participation and physical activity of a large cohort of VWD patients.
Methods
Patients were included from the “WiN study.” All patients completed a questionnaire on sports participation, physical activity, quality of life and bleeding symptoms (Tosetto bleeding score).
Results
From the 798 included patients, 474 had type 1, 301 type 2 and 23 type 3 VWD. The mean age was 39 ± 20 (standard deviation) years. Five hundred and fifty‐two patients (69.3%) participated in various types of sports. Type 3 VWD patients more often did not participate in sports due to fear of bleeding and physical impairment, respectively, OR = 13.24 (95% CI: 2.45‐71.53) and OR = 5.90 (95% CI: 1.77‐19.72). Patients who did not participate in sports due to physical impairment had a higher bleeding score item for joint bleeds 1.0 (±1.6) vs 0.5 (± 1.1) (P = 0.036). Patients with type 3 VWD and patients with a higher bleeding score frequently had severe limitations during daily activities, respectively, OR = 9.84 (95% CI: 2.83‐34.24) and OR = 1.08 (95% CI: 1.04‐1.12).
Conclusion
The majority of VWD patients participated in sports. Patients with type 3 VWD, a history of joint bleeds and a more severe bleeding phenotype frequently experienced limitations in sports participation and physical activities during daily life.
Keywords: physical activity, quality of life, sports, von Willebrand disease
1. INTRODUCTION
von Willebrand disease (VWD) is the most common inherited bleeding disorder.1 VWD is caused by reduced von Willebrand factor (VWF) levels (type 1), an abnormal function of VWF (type 2) or a complete absence of VWF (type 3).2, 3 Although VWD is clinically mainly characterized by mucocutaneous bleeding, all types of bleeding can occur.4, 5, 6 We previously found that adults and children with VWD have a lower health‐related quality of life (HRQoL) compared to the general population.6, 7, 8, 9 In adults, a more severe bleeding phenotype was associated with a lower HRQoL on the domains of physical functioning, and more often led to limitations due to impaired physical functioning.7 This is especially seen in patients with type 3, who typically have very low levels of FVIII and therefore have besides mucocutaneous bleeds, a more haemophilia‐like bleeding tendency, including joint and muscle bleeds.
Patients with VWD and other bleeding disorders including haemophilia are in several ways limited to perform physical activities and sports.10 They may have a higher risk of bleeding during physical activities and/or sports, especially high‐risk sports, and therefore, these patients may be less inclined to participate in physical activities and/or sports due to fear for such bleeding.11, 12 On the other hand, patients with bleeding disorders more often have a physical impairment due to (joint)bleeds and arthropathy and therefore could have a reduced physical activity in comparison with the general population.5, 11 It has been previously shown that although patients with haemophilia A and B participate as frequently in sports as the general population, they often participate in different types of sports.13, 14, 15 Patients generally avoided sports with a high risk of bleeding, such as soccer, as is advised by the World Federation of Hemophilia (WFH) and the American National Hemophilia Foundation (NFH).13, 16 Patients with haemophilia more often participated in swimming and cycling than the general population.13
It is well known that physical activity is important for the general health, as it is associated with great health benefits, such as a lower risk of cardiovascular disease, hypertension, obesity, diabetes, depression and osteoporosis.11 Several studies have been reported on sports participation and physical activity in haemophilia patients. No large studies have been performed on sports participation and physical activity in VWD patients.
Therefore, we aim to study the sports participation and physical activity of a large cohort of VWD patients. Secondly, we aim to identify subgroups of VWD patients who experience difficulties in sports participation and physical activity, in order to increase the awareness for the sports participation and physical activity of these patients.
2. MATERIALS AND METHODS
2.1. Participants
We included patients who participated in the “Willebrand in the Netherlands” (WiN) study; a nationwide cross‐sectional study in VWD patients in the Netherlands. Patients were included between 2007 and 2009. The inclusion criteria for the WiN study were haemorrhagic symptoms or a family history of VWD and historically lowest VWF:Ag and/or VWF:RCo ≤0.30 IU/mL and/or FVIII levels (FVIII:C) ≤0.40 IU/mL (for type 2N VWD). We excluded patients with other hemostatic disorders. Patients who were originally diagnosed with type 3 VWD were based on VWF propeptide <0.05 IU/mL classified as type 3 VWD (n = 23), whereas patients with VWF propetide ≥0.05 IU/mL were classified as type 1 VWD (n = 23).17 The study was approved by the Medical Ethical Committees of all participating centres. All patients signed informed consent.
2.2. Assessment methods
The assessment methods and laboratory measurements have been described in detail previously.4, 7, 18 Participants completed an extensive questionnaire, including questions on sports participation, physical activity, the short form‐36 (SF‐36) and a self‐administered version of the condensed Tosetto bleeding score.19, 20 Patients could report whether they participated in sports, and what kind of sports they did. Patients who did not participate in sports were additionally asked the reason why they did not perform sports. For physical activity, patients could report whether they did <1, 1‐2, 2‐4 hours or more than 4 hours of physical activity per week. Limitations in physical activity, general health status and happiness were derived from the SF‐36 questionnaire. Severe limitations during daily activity were defined as answering “yes, limited a lot” to the SF‐36 question on whether patients have physical limitations.
2.3. Bleeding risk of sports
Patients were asked to report the sports that they performed in the year prior to study inclusion. To classify sports based on their risk for bleeding, we used a modification of the taxonomy devised by the American National Hemophilia Foundation.21 We categorized sports in three groups instead of five groups, as has been done in prior studies.22 In Table 1, we present the bleeding risk category of some common sports in our population. Category 1 sports are sports with a low risk of bleeding (such as swimming). Category 2 sports are sports with an intermediate risk of bleeding (such as cycling). Category 3 sports are sports with a high risk of bleeding (such as soccer). Patients who performed several types of sports were categorized according to the sport with the highest risk of bleeding.
Table 1.
Risk of sports for bleeding
| Category | Sports |
|---|---|
| 1. Low risk | Swimming, walking, tai chi, dancing, table tennis, petanque (jeu de boules), golf, aerobics, watersports, steps, physiotherapy exercises, pool/snooker |
| 2. Intermediate risk | Yoga, cycling, climbing, school physical exercise lesson, running/jogging, volleyball, tennis, surfing, squash, karting, horse riding, skiing (cross‐country), korfball, gymnastics, badminton |
| 3. High risk | Wrestling, martial arts (except tai chi), boxing, soccer, skiing, (ice) skating, snowboarding, hockey, motorsport, rugby, racquetball, mountain biking |
2.4. Statistical methods
Categorical data are presented as frequencies and proportions. Continuous data are presented as median and interquartile range [IQR] or mean and (standard deviation [SD]).
Physical activity, sports participation and reasons not to participate in sports were compared between different types of VWD using a chi‐square test. Age was compared between different groups using an independent sample t test (central limit theorem). Binomial outcomes (sports vs no sport, high‐risk sport vs no high‐risk sport, etcetera.) were adjusted for age and sex using logistic regression analysis. The association between sports participation and bleeding history, and physical activity and bleeding history was only analysed for the total bleeding score, the bleeding score item for joint bleeds, the bleeding score item for muscle haematoma, the bleeding score item for cutaneous bleeding and the bleeding score item for menorrhagia. We analysed these associations using logistic regression analysis, in which we corrected for age and sex. For menorrhagia, we only corrected for age. We did not analyse other bleeding score items, because it is not biologically plausible that they are associated with sports participation or physical activity. The linear association between physical activity and general health status and happiness was investigated using the Mantel‐Haenszel test. We performed stepwise multivariate logistic regression analysis using the Wald test in a forward approach to identify variables that were independently associated with binomial outcomes. The variables that were included in the multivariate logistic analysis were age, sex, BMI and type of VWD.
Outcomes of logistic regression analyses are presented as odds ratio (OR) followed by the 95% confidence interval (CI). Statistical analyses were performed with SPSS IBM version 24.0 (IBM Corp., Armonk, NY, USA). A P‐value below 0.05 was defined as significant.
3. RESULTS
We included 798 patients of the total WiN study population of 837 patients. We excluded 39 patients due to missing data on physical activity and sports participation. Table 2 shows the baseline characteristics. Patients with type 3 VWD were younger than type 1 and type 2 VWD patients, respectively, 29 ± 20 (mean ± SD) vs 40 ± 20 and 38 ± 21 years (P = 0.044). Also, type 3 VWD patients had a higher total bleeding score than patients with type 1 and type 2 VWD, respectively, 19 [12‐23] vs 9 [5‐14] and 11 [7‐14] (P < 0.001; Table 2). Patients with type 1 VWD were more often female (n = 306 (64.6%) vs n = 160 (53.2%) in type 2 and n = 13 (56.5%) in type 3 VWD (P = 0.006)) and had more often blood group O (n = 294 (68.4%) vs n = 131 (50.4%) in type 2 and n = 7 (30.4%) in type 3 (P < 0.001; Table 2))
Table 2.
Baseline characteristics of the Von Willebrand disease patients
| Type 1 (n = 474) | Type 2 (n = 301) | Type 3 (n = 23) | |
|---|---|---|---|
| Age (y), mean ± SD | 40 ± 20* | 38 ± 21* | 29 ± 20* |
| Sex, female (%) | 306 (64.6%)* | 160 (53.2%)* | 13 (56.5%)* |
| BMI (kg/m2), mean ± SD | 24 ± 5.2 | 24 ± 5.1 | 23 ± 4.1 |
| Blood groups O (%) | 294 (68.4%)** | 131 (50.4%)** | 7 (30.4%)** |
| VWF levelsa | |||
| VWF:Ag (IU/mL) | 0.37 [0.22‐0.51] | 0.24 [0.16‐0.34] | 0.00 [0.00‐0.01] |
| VWF:CB (IU/mL) | 0.41 [0.21‐0.63] | 0.08 [0.06‐0.14] | 0.00 [0.00‐0.00] |
| VWF:Act (IU/mL) | 0.43 [0.22‐0.68] | 0.08 [0.04‐0.16] | 0.00 [0.00‐0.00] |
| FVIII:C (IU/mL) | 0.65 [0.47‐0.86] | 0.36 [0.27‐0.48] | 0.02 [0.01‐0.03] |
| Bleeding score | 9 [5‐14]** | 11 [7‐16]** | 19 [12‐23]** |
| Sports participation, number (%) | 334 (70.5%) | 204 (68.0%) | 14 (60.9%) |
| Physical activity per week, number (%)b | |||
| 0‐1 h | 92 (20.3%) | 48 (16.7%) | 7 (30.4%) |
| 1‐2 h | 112 (24.7%) | 76 (26.5%) | 4 (17.4%) |
| 2‐4 h | 129 (28.4%) | 85 (29.6%) | 6 (26.1%) |
| >4 h | 121 (26.7%) | 78 (27.2%) | 6 (26.1%) |
Data presented as median [interquartile ranges], unless otherwise specified;
Centrally measured at inclusion. Measurements available in 647 patients.
In type 1 and type 2 VWD, there were, respectively, 20 and 14 patients with missing data.
P < 0.05; ** P < 0.001 between types 1, 2 and 3 VWD.
3.1. Sports participation
From the total population, 552 VWD patients (69.3%) participated in sports in the previous months prior to inclusion in the study. In patients younger than 18, 18‐34, 35‐64 years and older than 65 years, respectively, 110 (72.4%), 131 (76.6%), 268 (66.2%) and 43 (62.3%) participated in sports. There was no difference in sports participation among the three types of VWD (Table 2, P = 0.520).
Most VWD patients participated in cycling (42%), followed by walking (36%) and swimming (24%; Figure 1). Twelve patients participated in martial arts, such as karate and kickboxing. In the total VWD study population, the percentage of patients who participated in low‐risk, intermediate‐risk and high‐risk sports was respectively 13.4%, 66.7% and 19.9%. The age of patients who participated in low‐risk, intermediate‐risk and high‐risk sports was 43 ± 20, 42 ± 19 and 26 ± 17 years, respectively (P < 0.001), indicating that high‐risk sports were performed more often by younger patients.
Figure 1.
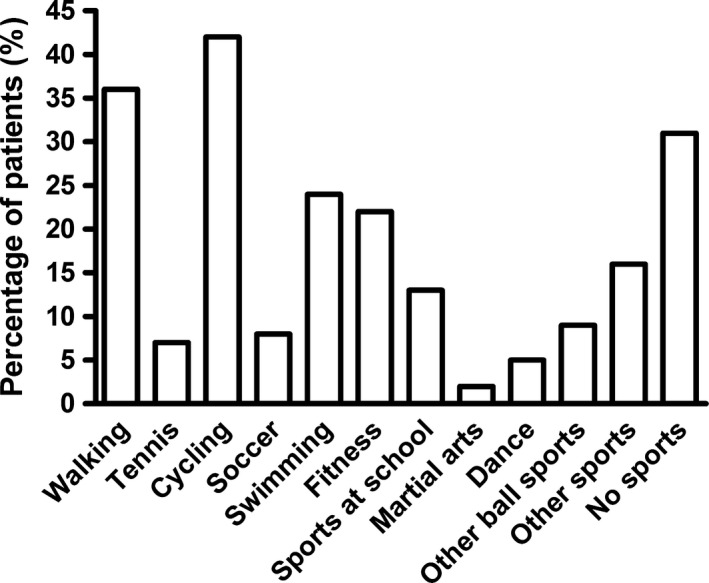
Types of sports performed by VWD patients
Patients who participated in sports had a higher bleeding score item for muscle haematoma 0.38 ± 0.95 vs 0.24 ± 0.75 (P = 0.023), which remained significant after correction for age and sex (OR = 1.26, 95% CI: 1.04‐1.53). In multivariate logistic regression analysis, BMI was independently associated with less frequent participation in sports (Table 3). Age and female sex were independently associated with less participation in high‐risk sports (Table 3).
Table 3.
Significant associations with binomial outcomes in multivariate logistic regression analysis
| Variable | Odds ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Sports participation | |||
| Body mass index | 0.96 | 0.93‐0.99 | 0.009 |
| Participation in high‐risk sports | |||
| Age | 0.96 | 0.95‐0.98 | <0.001 |
| Female sex | 0.24 | 0.14‐0.39 | <0.001 |
| No sports participation due to fear of bleeding | |||
| Age | 1.05 | 1.01‐1.09 | 0.013 |
| Type 3 VWD | 13.24 | 2.45‐71.53 | 0.003 |
| No sports participation due to physical limitations | |||
| Age | 1.03 | 1.01‐1.05 | 0.002 |
| Body mass index | 1.10 | 1.03‐1.17 | 0.004 |
| Type 3 VWD | 5.90 | 1.77‐19.72 | 0.004 |
| More than 4 h of physical activity per week | |||
| Body mass index | 0.95 | 0.91‐0.98 | 0.001 |
| Severe limitations to walk 1000 m | |||
| Age | 1.06 | 1.03‐1.08 | <0.001 |
| Female sex | 2.29 | 1.13‐4.63 | 0.021 |
| Body mass index | 1.14 | 1.08‐1.22 | <0.001 |
| Type 3 VWD | 4.51 | 1.08‐18.92 | 0.039 |
| Severe limitations to lift or to carry groceries | |||
| Age | 1.07 | 1.04‐1.09 | <0.001 |
| Female sex | 3.96 | 1.62‐9.72 | 0.003 |
| Type 3 VWD | 5.72 | 1.36‐24.05 | 0.017 |
| Severe limitations to climb up stairs | |||
| Age | 1.05 | 1.03‐1.08 | <0.001 |
| Body mass index | 1.13 | 1.06‐1.21 | <0.001 |
| Severe limitations to bow, to kneel or to bend | |||
| Age | 1.06 | 1.03‐1.08 | <0.001 |
| Body mass index | 1.14 | 1.07‐1.21 | <0.001 |
| Type 3 VWD | 9.81 | 2.78‐34.57 | <0.001 |
Outcomes of stepwise multivariate logistic regression analysis using the Wald test in a forward approach. The determinants that were included in all models were age, sex, body mass index and type of VWD. Only the determinants that were significantly associated with the binomial outcomes are presented.
3.2. Reason not to sport
Two hundred and forty‐six patients did not participate in sports, of whom 175 reported the reason why they did not perform sports. Most patients (47.4%) did not sport due to a lack of time, while 27.4% did not sport due to lack of motivation, 26.9% due to physical limitations, 6.9% due to fear of bleeding and 8.6% for other reasons. Patients with fear of bleeding were older (56 ± 17 vs 39 ± 20 years, P = 0.005), and had a higher total bleeding score (17 ± 7.0 vs 11 ± 7.2, P = 0.004), than other VWD patients. The total bleeding score remained higher after correction for age and sex (OR = 1.08, 95% CI: 1.00‐1.17). Multivariate logistic regression analysis revealed that age and type 3 VWD were independently associated with no sports participation due to fear of bleeding (Table 3).
Forty‐seven patients did not participate in sports due to physical limitations. Patients with physical limitations were older than other VWD patients, 52 ± 15 vs 35 ± 23 years (P < 0.001). The proportion of patients who did not participate in sports due to physical limitations was higher in type 3 VWD than in type 1 and type 2 VWD, respectively, 17.4% vs 6.1% and 4.7% (P = 0.041). They presented with a higher total bleeding score, a higher bleeding score for the joint bleeds item and a higher bleeding score item for menorrhagia, respectively, 15 ± 7.3 vs 11 ± 7.2 (P < 0.001), 1.0 ± 1.6 vs 0.5 ± 1.1 (P = 0.036) and 2.9 ± 1.2 vs 2.2 ± 1.5 (P = 0.003). After correction for age and sex, total bleeding score and bleeding score item for joint bleeds remained significantly higher in patients with physical limitations, respectively, OR = 1.05 (95% CI: 1.01‐1.09) and OR = 1.31 (95% CI: 1.07‐1.61). In multivariate logistic analysis age, BMI and type 3 VWD were independently associated with no participation in sports due to physical limitations (Table 3).
3.3. Physical activity
More than a quarter of the total population performed more than 4 hours of physical activity per week, whereas 18% was active for <1 hour per week (Table 1). Patients who performed more than 4 hours of physical activity per week were younger compared to patients with <4 hours of physical activity per week, 36 ± 21 vs 40 ± 19 years (P = 0.011). There was no difference in physical activity per week among the three types of VWD (Table 2; P = 0.723). There was no association between bleeding history and hours of physical activity (data not shown). Multivariate logistic analysis revealed that patients who did more than 4 hours of physical activity per week had a lower BMI (Table 3).
The percentage of patients aged 16 years and older who reported to have severe limitations in walking 100, 500 and 1000 m were, respectively, 2.4%, 5.4% and 8.7%. Patients with type 3 VWD had more frequent than other VWD patients severe limitations in walking distance: 500 m OR = 8.24 (95% CI: 1.96‐34.62) and 1000 m OR = 4.31 (95% CI: 1.07‐17.37), both corrected for age and sex (Figure 2). Furthermore, bleeding score item for joint bleeds was associated with severe limitations to walk 1000 m (OR = 1.24, 95% CI: 1.01‐1.53, corrected for age and sex). In multivariate logistic analysis age, sex, BMI and type 3 VWD were independently associated with severe limitations to walk 1000 m (Table 3).
Figure 2.
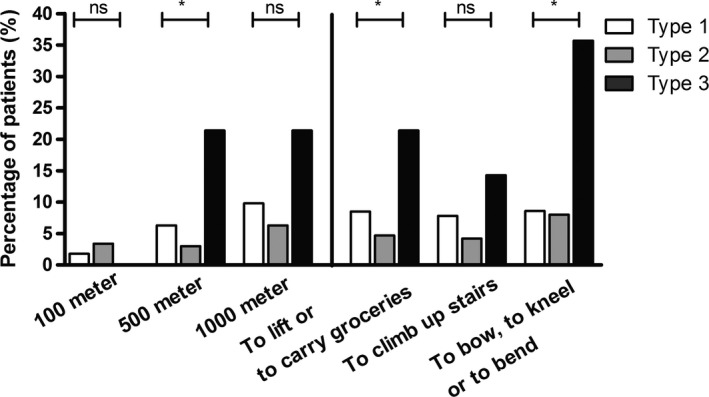
Proportion of VWD patients with severe limitations during daily life. Ns, not significant; *P < 0.05
3.4. Activities during daily life
The number of patients who had severe limitations to lift or to carry groceries, to climb up stairs, and to bow, to kneel or to bend was respectively 47 (7.4%), 42 (6.6%) and 57 patients (9.0%). Patients with type 3 VWD more often had severe limitations during these daily activities (Figure 2). After correction for age and sex, patients with type 3 VWD remained to have more often severe limitations to lift or to carry groceries and to bow, to kneel or to bend, respectively, OR = 5.80 (95% CI: 1.37‐24.56) and OR = 9.84 (95% CI: 2.83‐34.24).
A higher total bleeding score was associated with severe limitations to climb up stairs and severe limitations to bow, to kneel or to bend, respectively, OR = 1.07 (95% CI: 1.03‐1.12) and OR = 1.08 (95% CI: 1.04‐1.12), both corrected for age and sex. The bleeding score item for joint bleeds was also associated with severe limitations to climb up stairs (OR = 1.36, 95% CI: 1.09‐1.69, corrected for age and sex).
In multivariate logistic analysis age, female sex and type 3 VWD were independently associated with severe limitations to lift something up or to carry groceries (Table 3). Age and BMI were independently associated with severe limitations to climb up stairs (Table 3). Age, BMI and type 3 VWD were independently associated with severe limitations to bow, to kneel or to bend (Table 3).
3.5. Physical activity, general health status and happiness
We found a linear association between more hours of physical activity per week and a better general health status (P < 0.001). For instance, from the patients who did more than 4 hours of physical activity per week, 58 patients (37.2%) scored their health status as very good or excellent, whereas in patients who did <1‐hour physical activity per week only 24 patients (20%) scored their health status as very good or excellent. Moreover, in patients who did more than 4 hours of physical activity per week, only 20 patients (12.8%) scored their general health as moderate or bad, whereas in patients who did <1‐hour physical activity per week 34 patients (28.3%) scored their health status as moderate or bad. Additionally, we found a linear association between more hours of physical activity per week and how happy patients felt in the previous 4 weeks before study inclusion (P < 0.001). Furthermore, patients who participated in sports scored their health status better (P < 0.001) and tended to feel more happy the previous 4 week before inclusion in the study (P = 0.082), than patients who did not participate in sports.
4. DISCUSSION AND CONCLUSION
This study shows that although a large proportion of VWD patients participated in various types of sports, type 3 VWD patients are limited in sports participation in several ways. Patients with type 3 VWD, a more severe bleeding phenotype and a history of joint bleeds more often did not participate in sports because of fear of bleeding or because of physical limitations. Moreover, these patients frequently had severe limitations in walking distance and severe limitations during daily activities. Lastly, patients who did more physical activities and patients who participated in sports scored higher on general health status and felt more happy in the month prior to study inclusion, than other VWD patients.
The sports participation rate in VWD patients in our study is comparable to the general sports participation rate in the Netherlands.13 In males ageing 16‐55 years, the types of sports in which VWD patients participated were very comparable to the types of sports in the general population (Figure 3). Even participation in a high‐risk sport as soccer was comparable between VWD patients and the general population. This is in contrast to patients with severe haemophilia, who participated less frequently in high‐risk sports such as soccer, while they more often participated in swimming (Figure 3).13 This is in line with the recommendation on sports participation in haemophilia patients, as recently reviewed by Howell et al16
Figure 3.
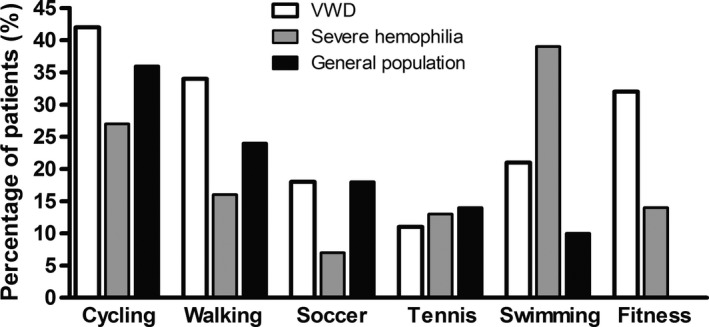
Participation in various types of sports in males aged 16‐55 y with VWD and haemophilia compared to the general population. Data on haemophilia and general population derived from Heijnen et al13
In the healthy population and haemophilia patients, sports participation and physical activity are associated with a better quality of life and may reduce the frequency of spontaneous bleeding due to improved strength and fitness.16, 23, 24, 25 In accordance, we also found that VWD patients who participated in sports and patients who were more physically active scored their general health status better and felt more happy, than other VWD patients. This may indicate that sports participation and physical activity are not only beneficial to physical health in patients with bleeding disorders, but also for mental health.
Patients who did not participate in sports due to fear of bleeding or physical limitations more often had type 3 VWD and also had a more severe bleeding phenotype. This was expected, given the more severe bleeding phenotype in patients with type 3 compared to type 1 and 2 VWD. In our population, VWD patients with physical limitations experienced more severe joint bleeds in the past. In a previous study, we found that VWD patients with joint bleeds in the past had more often joint damage and chronic joint pain than other VWD patients.5, 26 This may explain why these patients participated less often in sports. Furthermore, women with menorrhagia more often did not participate in sports due to physical limitations. This may indicate that in these women, menorrhagia has a significant role on their social activities. It is important for patients with type 3 VWD, patients with a high bleeding score and patients with joint bleeds in the past, to be informed on the relevance of sports participation and possibilities of participating in sports with a low bleeding risk (such as swimming), and to advise sports that fit their physical abilities.16, 27 In women with menorrhagia, it is important to be aware of the possible impact of menorrhagia on their social life.
We did not find an association between VWF levels, type 2 VWD and sports participation or physical activity (data partly not shown). As expected, this means that a severe bleeding history is better correlated with sports participation and physical activity than VWF levels. Moreover, it indicates that only the most severe VWD patients (ie, type 3 VWD and patients with a severe bleeding phenotype) are limited in sports participation and physical activity, while patients with a moderate bleeding tendency (type 2 VWD) probably experience less limitations.
The strength of this study is that it is the first study to investigate sports participation and physical activity of VWD patients. We included a large number of VWD patients. Therefore, we were able to compare our results within the types of VWD. We also compared our findings with previous reports in haemophilia patients and the general population in the Netherlands. Moreover, we were able to assess the association between sports participation and physical activity and the bleeding history of VWD patients.
The primary limitation of this study is that we did not use a validated physical activity questionnaire. However, the questions that were asked about sports participation and physical activity per week were similar to the questionnaire used in the Hemophilia in the Netherlands (HiN‐5) study.13 Moreover, we used validated SF‐36 questions to quantify and to describe physical activity during daily life. Secondly, the outcomes on sports participation and physical activity are patient‐reported. Since no clear definitions for sports and physical activity exist, it is possible that a patient confused these terms. Nevertheless, there is no reason to assume that the type of VWD or the bleeding risk is associated with the interpretation of the questions; therefore, it is unlikely that this potential minor deviancy influenced the results. Another limitation of this study is that we did not have detailed information on how intense or frequent sports were performed. Lastly, we did not include a control group.
In conclusion, this is the first study on the physical activity and sports participation of patients with VWD. In general, VWD patients participate as frequent and in the same types of sports as the general population. However, patients with type 3 VWD, a more severe bleeding phenotype, and a history of joint bleeds participate less frequently in sports due to fear of bleeding or physical impairment. Moreover, these patients frequently have limitations in walking distance and in physical activities during daily life.
DISCLOSURES
F Atiq received research support from CSL Behring. FWG Leebeek received research support from CSL Behring and Shire for performing the Willebrand in the Netherlands (WiN) study, and is consultant for UniQure, Novo Nordisk and Shire, of which the fees go to the institution, and is a member of a DSM board for Roche. J Eikenboom received research support from CSL Behring and he has been a teacher on educational activities of Roche. KPM van Galen received unrestricted research support from CSL Behring and Bayer. EP Mauser‐Bunschoten received unrestricted research/educational support from CSL Behring, Bayer, Baxter, Grifols, Novo Nordisk, Pfizer, Biotest and Sanquin. JG van der Bom has received unrestricted research/educational funding for various projects from the following companies: Bayer Schering Pharma, Baxter, CSL Behring, Novo Nordisk, and Pfizer. In addition, she has been a consultant to Baxter and Pfizer, and she has been a teacher on educational activities of Bayer Schering Pharma. MH Cnossen has received unrestricted research/educational funding for various projects and travel grants from NOW‐ZonMW, Innovatiefonds and the following companies: Pfizer, Baxter, Bayer Schering Pharma, CSL Behring, Novo Nordisk and Novartis, and serves as a member on steering boards of Roche and Bayer. K Fijnvandraat is a member of the European Hemophilia Treatment and Standardization Board sponsored by Baxter, has received unrestricted research grants from CSL Behring and Bayer, and has given lectures at educational symposiums organized by Pfizer, Bayer and Baxter. K Meijer received research support from Bayer, Baxter, Sanquin and Pfizer; speaker fees from Bayer, Sanquin, Boehringer Ingelheim, BMS and Aspen; consulting fees from Uniqure. BAP Laros‐van Gorkom has received unrestricted educational grants from Baxter and CSL Behring. None of the other authors has a conflict of interest to declare.
AUTHOR CONTRIBUTION
F. Atiq designed the study, performed statistical analysis, interpreted data and wrote the manuscript. E. Mauser‐Bunschoten, J. Eikenboom, K. van Galen, K. Meijer, J. de Meris, M. Cnossen, E. Beckers, B. Laros‐van Gorkom, L. Nieuwenhuizen, J. van der Bom and K. Fijnvandraat designed the study, interpreted data and critically revised the manuscript. F. Leebeek conceived of and designed the study, interpreted data and critically revised the manuscript. All authors gave their consent to the final version of the manuscript.
Supporting information
ACKNOWLEDGEMENTS
This study was supported (in part) by research funding from the Dutch Hemophilia Foundation (Stichting Haemophilia) and CSL Behring (unrestricted grant).
Atiq F, Mauser‐Bunschoten EP, Eikenboom J, et al.; for the WiN study group . Sports participation and physical activity in patients with von Willebrand disease. Haemophilia. 2019;25:101–108. 10.1111/hae.13629
The WiN study group present in AppendixS1 in Supporting Information.
REFERENCES
- 1. Leebeek FW, Eikenboom JC. Von Willebrand's disease. N Engl J Med. 2016;375:2067‐2080. [DOI] [PubMed] [Google Scholar]
- 2. Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4:2103‐2114. [DOI] [PubMed] [Google Scholar]
- 3. Von RF. Willebrand disease: pathogenesis and management. Thromb Res. 2013;131(Suppl 1):S47‐S50. [DOI] [PubMed] [Google Scholar]
- 4. de Wee EM, Sanders YV, Mauser‐Bunschoten EP, et al. Determinants of bleeding phenotype in adult patients with moderate or severe von Willebrand disease. Thromb Haemost. 2012;108:683‐692. [DOI] [PubMed] [Google Scholar]
- 5. van Galen KP, Sanders YV, Vojinovic U, et al. Joint bleeds in von Willebrand disease patients have significant impact on quality of life and joint integrity: a cross‐sectional study. Haemophilia. 2015;21:e185‐e192. [DOI] [PubMed] [Google Scholar]
- 6. Sanders YV, Fijnvandraat K, Boender J, et al. Bleeding spectrum in children with moderate or severe von Willebrand disease: Relevance of pediatric‐specific bleeding. Am J Hematol. 2015;90:1142‐1148. [DOI] [PubMed] [Google Scholar]
- 7. de Wee EM, Mauser‐Bunschoten EP, Van Der Bom JG, et al. Health‐related quality of life among adult patients with moderate and severe von Willebrand disease. J Thromb Haemost. 2010;8:1492‐1499. [DOI] [PubMed] [Google Scholar]
- 8. de Wee EM, Fijnvandraat K, de Goede‐Bolder A, et al. Impact of von Willebrand disease on health‐related quality of life in a pediatric population. J Thromb Haemost. 2011;9:502‐509. [DOI] [PubMed] [Google Scholar]
- 9. Barr Ronald D, Sek J, Horsman J, et al. Health status and health‐related quality of life associated with von Willebrand disease. Am J Hematol. 2003;73:108‐114. [DOI] [PubMed] [Google Scholar]
- 10. Kumar M, Lambert MP, Breakey V, et al. Sports participation in children and adolescents with immune thrombocytopenia (ITP). Pediatr Blood Cancer. 2015;62:2223‐2225. [DOI] [PubMed] [Google Scholar]
- 11. Negrier C, Seuser A, Forsyth A, et al. The benefits of exercise for patients with haemophilia and recommendations for safe and effective physical activity. Haemophilia. 2013;19:487‐498. [DOI] [PubMed] [Google Scholar]
- 12. Sherlock E, O'Donnell JS, White B, Blake C. Physical activity levels and participation in sport in Irish people with haemophilia. Haemophilia. 2010;16:e202‐e209. [DOI] [PubMed] [Google Scholar]
- 13. Heijnen L, Mauser‐Bunschoten EP, Roosendaal G. Participation in sports by Dutch persons with haemophilia. Haemophilia. 2000;6:537‐546. [DOI] [PubMed] [Google Scholar]
- 14. Buxbaum NP, Ponce M, Saidi P, Michaels LA. Psychosocial correlates of physical activity in adolescents with haemophilia. Haemophilia. 2010;16:656‐661. [DOI] [PubMed] [Google Scholar]
- 15. Groen WG, Takken T, van der Net J, Helders PJ, Fischer K. Habitual physical activity in Dutch children and adolescents with haemophilia. Haemophilia. 2011;17:e906‐e912. [DOI] [PubMed] [Google Scholar]
- 16. Howell C, Scott K, Patel DR. Sports participation recommendations for patients with bleeding disorders. Transl Pediatr. 2017;6:174‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanders YV, Groeneveld D, Meijer K, et al. von Willebrand factor propeptide and the phenotypic classification of von Willebrand disease. Blood. 2015;125:3006‐3013. [DOI] [PubMed] [Google Scholar]
- 18. Atiq F, Meijer K, Eikenboom J, et al. Comorbidities associated with higher von Willebrand factor (VWF) levels may explain the age‐related increase of VWF in von Willebrand disease. Br J Haematol. 2018;182:93‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tosetto A, Rodeghiero F, Castaman G, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM‐1 VWD). J Thromb Haemost. 2006;4:766‐773. [DOI] [PubMed] [Google Scholar]
- 20. Tosetto A, Castaman G, Rodeghiero F. Bleeding scores in inherited bleeding disorders: clinical or research tools? Haemophilia. 2008;14:415‐422. [DOI] [PubMed] [Google Scholar]
- 21. Anderson A. Playing it safe. National Hemophilia Foundation. 2017. [Google Scholar]
- 22. Broderick CR, Herbert RD, Latimer J, et al. Association between physical activity and risk of bleeding in children with hemophilia. JAMA. 2012;308:1452‐1459. [DOI] [PubMed] [Google Scholar]
- 23. Seuser A, Bohm P, Wermes C. Early orthopaedic challenges in haemophilia patients and therapeutic approach. Thromb Res. 2014;134(Suppl 1):S61‐S67. [DOI] [PubMed] [Google Scholar]
- 24. Von Mackensen S. Quality of life and sports activities in patients with haemophilia. Haemophilia. 2007;13(Suppl 2):38‐43. [DOI] [PubMed] [Google Scholar]
- 25. Tiktinsky R, Kenet G, Dvir Z, et al. Physical activity participation and bleeding characteristics in young patients with severe haemophilia. Haemophilia. 2009;15:695‐700. [DOI] [PubMed] [Google Scholar]
- 26. van Galen K, de Kleijn P, Foppen W, et al. Long‐term impact of joint bleeds in von Willebrand disease: a nested case‐control study. Haematologica. 2017;102:1486‐1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seuser A, Boehm P, Kurme A, Schumpe G, Kurnik K. Orthopaedic issues in sports for persons with haemophilia. Haemophilia. 2007;13(Suppl 2):47‐52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


