Figure 1.
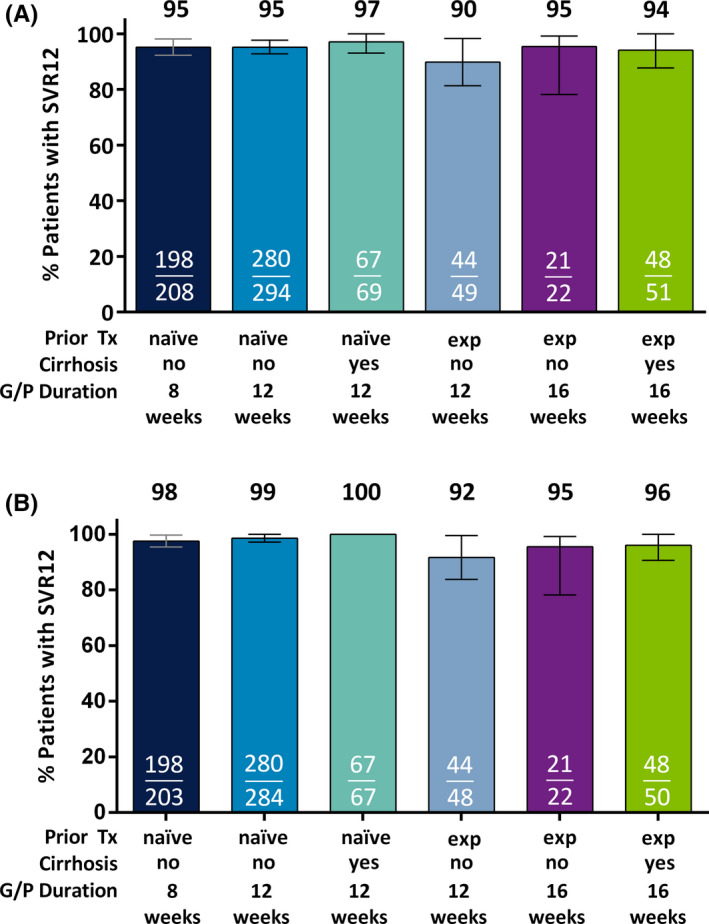
Efficacy of G/P in patients with HCV genotype 3 infection. Patients with HCV genotype 3 were grouped based on prior treatment experience, cirrhosis status and duration of G/P treatment received. Rates of sustained virologic response at post‐treatment week 12 are shown in the (A) intention‐to‐treat (ITT) population, which includes all patients who received at least one dose of study drug, and (B) modified ITT population, which excludes those patients in the ITT population with premature discontinuation, loss to follow‐up or nonadherence to the study drug. For SVR12 rates less than 100%, confidence intervals were calculated at 95% using the normal approximation to the binomial distribution. Tx, treatment; exp, experienced; wks, weeks, SVR12, sustained virologic response at post‐treatment week 12; G/P, glecaprevir and pibrentasvir
