Figure 6. Prediction of therapeutic benefit in subsets of patients.
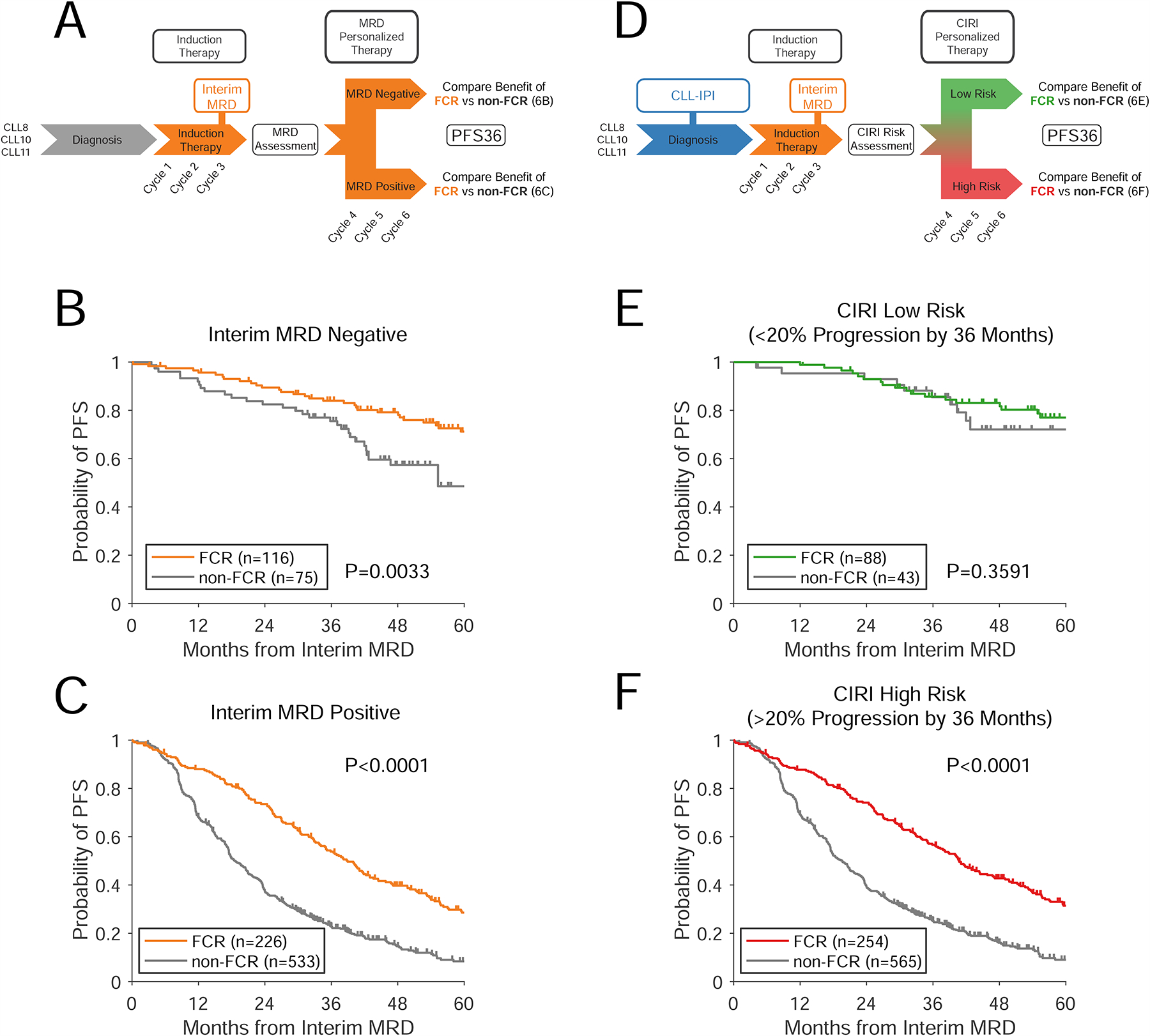
A) A schema for using interim MRD to guide therapy in CLL. Patients receive a period of induction therapy; after which interim MRD is assessed. The effect of different types of therapy can then be assessed in patients with interim MRD negative or positive disease. Here, we assessed this paradigm using patients from the CLL8, CLL10, and CLL11 clinical trials receiving chemo-immunotherapy (i.e., FCR, BR, R-chlorambucil, G-chlorambucil), blinding ourselves to the choice of therapy over the first 3 cycles. B–C) Kaplan-Meier estimates show the benefit of therapy with FCR vs alternative therapies for progression-free survival in interim MRD negative patients (Panel B) and interim MRD positive patients (Panel C). Survival is landmarked from the time of interim MRD assessment. D) A schema for using CIRI-CLL to discover predictive biomarkers to guide therapy. Patients receive a pretreatment risk-prediction (using the CLL-IPI), and then receive a period of induction therapy. At this point, interim MRD is assessed, allowing quantitative integration with CIRI. E–F) Kaplan-Meier estimates show the PFS of patients receiving FCR vs alternative therapies in patients with CIRI risk < 20% (Panel E) and patients with CIRI risk > 20% (Panel F). See also Figures S5–6.
