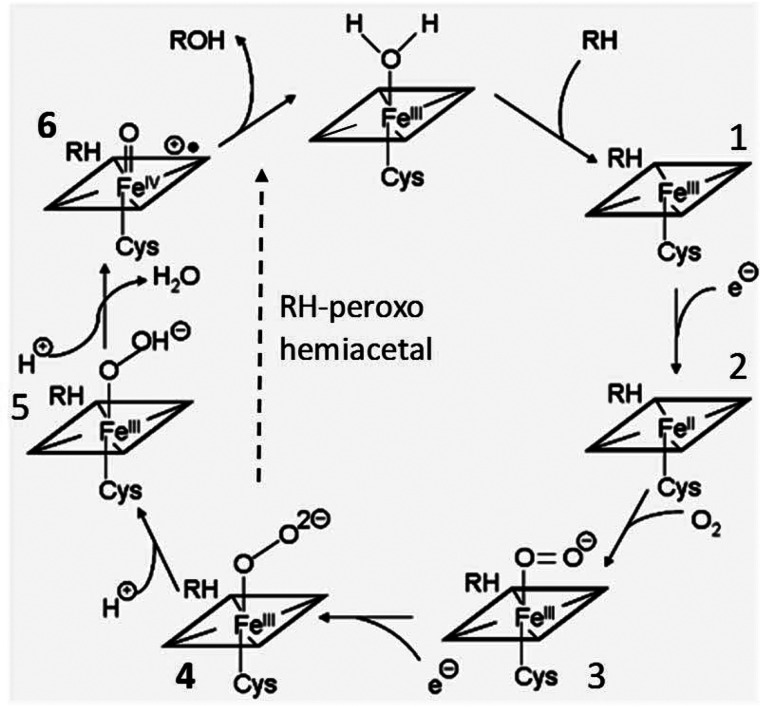
Figure 2.
The cytochrome P450 catalytic cycle. Binding of the substrate (1) displaces the water molecule from the sixth (distal) coordination site of the ferric heme iron, changing its spin state from hexa-coordinated low to penta-coordinated high, increasing its redox potential, and facilitating the first electron transfer from the protein redox partner (2). The ferrous iron complex binds molecular oxygen (3), which triggers acceptance of the second electron producing the nucleophilic ferric peroxo anion (4) (compound 0, or Fe3+–O2−). The ferric peroxo complex is then protonated to form the ferric hydroperoxo state (5), the second protonation of the distal oxygen atom causing the O-O bond scission, release of a water molecule, and generation of a highly reactive electrophilic Fe4+-oxo cation radical (FeO3+, or compound I) (6). The compound I mechanism requires two protons delivered from the protein surface to the iron-bound dioxygen via the active proton delivery network. In the compound 0 mechanism, the dioxygen abstracts protons from the substrate, forming a peroxo-hemiacetal intermediate and, thus, being independent of proton delivery.