Abstract
This article explores the multifactorial relationship between mastication and cognition, with a focus on dementia. Older persons, especially those with dementia, are at great risk of suffering from oral health problems such as orofacial pain and loss of natural teeth. A possible explanation could be that the cognitive and motor impairments resulting from dementia cause a decrease in self‐care and as such, a worsening of oral health. An alternative explanation is that cognition and oral health influence each other. Animal studies show that a decrease in masticatory activity, for example, due to a soft diet or loss of teeth, causes memory loss and neuronal degeneration. The relationship between mastication and cognition has also been researched in human studies, but a cause‐effect relationship has not been proven. It is likely that multiple factors play a role in this relationship, such as self‐care, nutrition, stress and pain.
1. INTRODUCTION
Recent systematic literature research has shown that older people run a high risk of oral problems such as orofacial pain and the loss of dental elements.1 The oral health of nursing home residents is poor, particularly among older persons with dementia. Research indicates that 66% of psycho‐geriatric nursing home residents have serious periodontitis, a proportion almost three times higher than it is among nursing home residents without dementia.2 Older persons with dementia also have significantly more remaining roots (radices relictae), more coronal caries and more root caries than those without dementia.1 In 2007, a large research study was carried out in Finland which looked at 2320 people aged 55 and over; it revealed that even in this relatively young group, older people with dementia also had more caries and poorer denture hygiene compared to healthy persons.3 Older people with a cognitive impairment were more likely to report problems chewing hard food and were more likely to be entirely edentate and to wear no dental prosthesis than those with no cognitive impairment. Within the group of older persons with a cognitive impairment, those wearing a dental prosthesis regularly suffered from denture‐related ulcers (ie, sore spots) as a result of this dental prosthesis.3 An intuitive response to these findings might be that dementia itself leads to reduced self‐care, and an accompanying decline in daily oral hygiene, with worsened oral health and an increased risk of tooth loss, as a result. It might also be the case, however, that a certain reciprocity is at work, in which cognitive ability and oral health influence one another. In that case, a vicious circle could arise in which dementia worsens and dental health simultaneously declines. This raises the likelihood of all kinds of painful oral disorders.4 It then also becomes more likely that the patient eats softer food and displays reduced masticatory activity.
Animal studies have shown that in older age groups reduced masticatory activity, for instance, through the eating of crushed food, leads to the loss of spatial memory, reduced learning capacity, neuro‐endocrinal changes and hippocampal degeneration.5 The hippocampus is a brain area that, among other functions, is important to memory.5 The relationship between mastication and cognitive function has also been studied in human populations, but these studies have not clarified any causalities involved, possibly because of the large diversity in research populations and methods. Nevertheless, some important insights have been gained. The aim of this article was to provide an overview of recent insights into the relationship between mastication and cognition, with a focus on older people with dementia.
2. ANIMAL RESEARCH
A longitudinal study of mice examined the effect of food hardness on spatial memory and learning ability.6 A first group of animals was given hard food pellets; a second group was first given hard food, and then powdered food; and a third, “rehabilitated” group was first given hard food, then powdered food and finally hard food again. The experiment was carried out on adult and aged animals, and under two different sets of conditions: an “impoverished environment” (a standard plastic cage) and an “enriched environment” (a larger cage containing a variety of toys). At the end of each experiment, the animals’ spatial learning ability was tested by means of a water maze. The results of this experiment were interesting: under impoverished conditions, ageing itself led to poorer performance. A soft diet also led to poorer performance, both in adult and in aged mice. An enriched environment had a positive influence on performance: adult mice from the enriched environment performed better than the comparable control group from the standard environment. In the adult (but not the aged) group, rehabilitation with hard food led to improved performance, irrespective of the living environment. The older, rehabilitated mice recovered to normal performance levels only if they lived in the enriched environment; rehabilitated aged animals living in the standard environment did not display this recovery.6
Comparable results were seen in another study.7 In aged mice, molars in the upper jaw were extracted and the mice were kept for 3 weeks in either “standard” or “enriched environment” cages. The behavioural effects of these actions were then assessed using a water maze. The proliferation and differentiation of newborn neurons were assessed post‐mortem, along with the development and retention of existing neurons. Molar loss turned out to have a negative influence on brain function: molarless mice displayed poorer spatial memory and learning ability. This group also developed fewer new neurons and retained fewer existing neurons than did fully dentate mice. An enriched environment had a positive influence: mice from the enriched environment had better spatial memory, greater learning ability, more newborn neuron proliferation and greater neuron retention than the mice from the standard environment. There was also a significant interaction effect from the two factors of “environment” and “molar loss” with regard to the survival of existing neurons: a molarless mouse in the standard environment retained fewer neurons than did a molarless mouse in the enriched environment, and no <25% fewer than a mouse in the enriched environment that had retained its teeth. In the enriched environment, a molarless mouse did not, incidentally, show significantly different scores than a fully dentate mouse. The nature of the environment therefore appeared to cancel out the negative effect of molar extraction in this study.7
In 2016, an animal study showed that the effect of an enriched environment in preventing Alzheimer's disease in mice arose principally through the degree of physical activity and that solitary running in a wheel had the same protective effect as living in an enriched environment.8 Mastication could be regarded as a form of physical activity,9 one which also stimulates cerebral blood flow.10 In many cases, the restoration of masticatory activity is not brought about simply by a modification of diet; dental intervention is often required to restore masticatory ability. This matter was examined in an animal study11 in which the crowns of the upper molars were artificially abraded in a group of aged mice. A water maze was then employed to determine the behavioural effects of this intervention, and neural plasticity (ie, responsive adaptations in the organisation of cell structures in the brain) was established post mortem. The mice all lived in “standard” conditions. The results showed that molar abrasion was linked to poorer spatial memory and reduced neural plasticity, especially in the hippocampus, compared to the control group. It also became clear that the longer the mouse had abraded molars, the more prominent this worsening became. A number of mice were given a new molar crown after 10 days. The restored masticatory function resulted in improved learning ability and neural plasticity, although not to the levels of mice that had retained their original molars. In mice whose molars were restored in this way, spatial memory improved up to a level of 74% of the control group of mice who had retained their own teeth. Compared with the group that had not received crowns, this was an improvement of 150%.11 An unfortunate shortcoming of this study is that it measured only the effect of reduced masticatory ability, without regard to the possible presence and role of pain during the intervention.
3. HUMAN RESEARCH
Although animal studies are extremely interesting and can indicate directions for follow‐up research, their findings cannot simply be extrapolated to the human population. For this reason, studies of human subjects are needed, and this necessitates a different approach. A number of studies on the effects of mastication on cognition have been carried out in healthy adults. Patient studies have also been done, for instance, with older persons with dementia.
3.1. MASTICATION AND COGNITION IN COGNITIVELY INTACT ADULTS
Research studies of human subjects have shown that chewing of a piece of gum has an acute and positive effect on working memory.12 Even mood might be positively influenced by chewing gum: in a small study, it was found that mildly depressed patients who received medicinal treatment benefitted from complementary (sugar‐free, flavourless) chewing gum therapy to relieve somatic symptoms of depression such as appetite loss.13 Although these are interesting and encouraging results, some studies have failed to replicate them,14 and others have even found negative effects of gum chewing on cognitive performance measures.15 Moreover, this kind of research is often performed only on young adult subjects even though age‐dependent differences have been found in the response to mastication, for instance, in cerebral blood flow.16 The fact that excessive gum chewing can lead to pain and fatigue in the jaw muscles17 means that some restraint should always be exercised when recommending gum chewing. Potentially, positive long‐term effects of gum chewing have not been adequately demonstrated.
A large‐scale Swedish population study of older community‐dwelling people (n = 557, aged 77 or older) showed that respondents whose cognitive function screening test score indicated the possible onset of dementia also had more difficulty chewing hard food such as apples.18 The number of natural (as opposed to prosthetic) dental elements had no influence on this outcome. The authors concluded that tooth loss did not necessarily lead to cognitive impairment as long as there were no masticatory difficulties.18 The relationship between cognitive function and masticatory ability has also been studied in a population of older, cognitively intact, independently living Dutch persons wearing full dental prostheses.19 Cognitive function was measured using a neuropsychological test battery. Masticatory function was assessed by measuring maximum mouth opening, maximum lateral and forward jaw movement, maximum bite force and the number of occluding pairs. Complaints about the stomatognathic system, with regard to orofacial pain and headache, were assessed using the Axis‐II questionnaire by the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD).20 By combining the scores for various neuropsychological tasks, an “episodic memory” domain and an “executive function” domain were created.19 Executive function is an important concept within neuropsychology and describes goal‐directed behaviour,21 including working memory, inhibition, planning and attention.22 The prefrontal cortex is particularly strongly associated with executive function, although other sub‐cortical areas and networks in the brain are also involved in executive function.21 The Dutch study revealed that among full dental prosthesis wearers, performance in executive function was poorer when there were also complaints about masticatory function.19 Performance in episodic memory was positively associated with bite strength. Backward multiple regression analysis was then employed in order to analyse the influence of the different variables involved. This showed that 19% of episodic memory function could be predicted by jaw mobility and bite strength and that 22% of performance variation in executive function was related to complaints about masticatory function.19
Another study used imaging techniques to map the effects of mastication on cerebral blood flow and revealed that the frontal cortex, in particular, became more active during mastication.23 The effect of mastication on the activation of these areas was stronger in older individuals.16 It could therefore be the case that mastication stimulates cerebral blood flow, particularly in the frontal cortex, which could then have an influence on executive function and therefore on cognitive performance. In a small clinical study (n = 9) of middle‐aged test subjects with unilateral loss of the first and second inferior molars, the electromyographic (EMG) activity of the left and right masseter muscles was measured during a clench task, and pupil diameters were measured both at rest and during a sensory search task.24 Pupil diameter increases in reaction to cognitive tasks requiring memory and attention (mydriasis), a phenomenon which is used as an indicator of mental arousal in psychophysiological experiments.25 A digit retrieval task was also given, in which as many numbers as possible had to be identified on a 10 × 10 matrix of digits during 30 seconds.24 Participants were fitted with dental implants, and then, three different situations were studied: mouth open, without crowns on the implants; mouth closed, without crowns on the implants; and mouth closed, with crowns on the implants. During the digit retrieval task, all participants displayed asymmetry both in EMG activity and in pupil diameter (anisocoria). The placement of implants restored dental occlusion, and both asymmetric EMG activity and the anisocoria disappeared.24 The second situation (mouth closed, no crown) yielded the poorest intellectual performance, and the third situation (mouth closed, with crowns) yielded the best intellectual performance. The restoration of occlusion seemed to contribute towards the restoration of cognitive function. Pupil diameter asymmetry however remained, to a smaller degree, as long as 6 months after crowns had been fitted to the implants. A possible explanation for this might be that sensory asymmetry resulting from the loss of input from periodontal mechanoreceptors was not, or not entirely, removed by treatment with implants.24
3.2. MASTICATION AND COGNITION IN OLDER PEOPLE WITH DEMENTIA
The relationship between oral health, masticatory function and cognitive function has also been studied in older people with dementia. In a group of 60 community‐dwelling persons, the oral health of Alzheimer's disease (AD) patients with mild, moderate or serious cognitive impairment was compared with the oral health of a control group.26 Subjective oral health was determined using the Geriatric Oral Health Assessment Index (GOHAI) questionnaire; a higher GOHAI score implies a more positive perception by the respondent of their oral health. Objective oral health was determined by oral examination, which looked at the presence and condition of natural and prosthetic dental elements. The Decayed‐Missing‐Filled Teeth (DMFT) index was determined, and the presence of plaque and tartar was assessed. The GOHAI scores of all groups were comparable. This is remarkable, because the AD group had poorer objective oral health: they had fewer natural teeth and a higher DMFT score, and this group was more likely to suffer from an oral health disorder. The AD group with serious cognitive impairment had the highest GOHAI scores, that is, had the most positive perception of their own oral health. In answering the GOHAI questions, study participants with AD were assisted by their caregivers. The authors concluded that, in terms of their own subjective perception, participants with and without AD were positive about their own oral health, but that those participants with AD actually showed oral health problems that increased with their level of cognitive impairment. This shows that neither these participants nor their caregivers were particularly skilled at assessing oral health.26
In another study, the masticatory function (amongst other variables) of 29 older people with dementia was compared with that of 22 cognitively healthy matched subjects.27 Masticatory function was measured as the ability to mix a two‐coloured chewing gum. The number of natural and prosthetic dental elements was similar in both groups. The persons with dementia turned out to have three times as much visible plaque, to be more dependent on others in the execution of daily life activities and to be more frequently undernourished than the control group. They also performed less well at the two‐colour gum‐mixing task. The authors concluded that masticatory function appeared to be more closely related to cognitive impairment than to the number of dental elements. The loss of masticatory ability was explained by a reduction in motor skills resulting from dementia.27 In a group of 114 older persons with dementia who were living in a nursing home or who regularly visited a day centre, masticatory ability was also measured using the two‐colour gum‐mixing approach. Their cognitive function was measured using a comprehensive neuropsychological test battery.28 Of the eight neuropsychological tests that were carried out, two turned out to have a significant relationship with masticatory function, namely “general cognition” and “word fluency”.28 In a word fluency test, the participant was asked—in the course of a protocol‐directed interview—to name as many words as possible within a certain category, within a certain short period of time. This task appeals to memory, planning and inhibition, and is therefore a good indicator of executive function. Only half of the 114 participants performed the two‐colour chewing gum task. The most common reason for nonparticipation was concern among care staff about the possible agitation of the participants.28 Of the 58 participants who performed the two‐colour gum‐mixing task, 56 also completed the general cognition task and 51 completed the word fluency task. Correlations were investigated within these subgroups and it turned out that those participants who performed better at the gum‐mixing task also displayed better cognitive performance, as shown by the general cognition test and the word fluency test.28
4. UNDERLYING MECHANISMS
A variety of underlying mechanisms can be put forward to explain the relationship between masticatory ability and cognitive function.
It is possible, for instance, that effective mastication of food leads to improved nutrition and that this contributes towards preservation of cognition.5, 27 Another hypothesis is that mastication raises cerebral arousal levels,24 or that, as a physical activity, it contributes towards an enriched environment.7 It is also possible that active mastication contributes towards the reduction in stress and/or pain12; for instance, an animal study found that gnawing and chewing during a stressful situation kept cognitive scores at the same level as they were in nonstressed control animals, while a stressed group that was prevented from gnawing and chewing showed significantly lower cognitive scores than the control group.29 It is also known that stress, whether or not caused by pain, has a negative influence on cognition.5 Figure 1 drafts a possible interaction model linking mastication, cognition, stress and pain.
Figure 1.
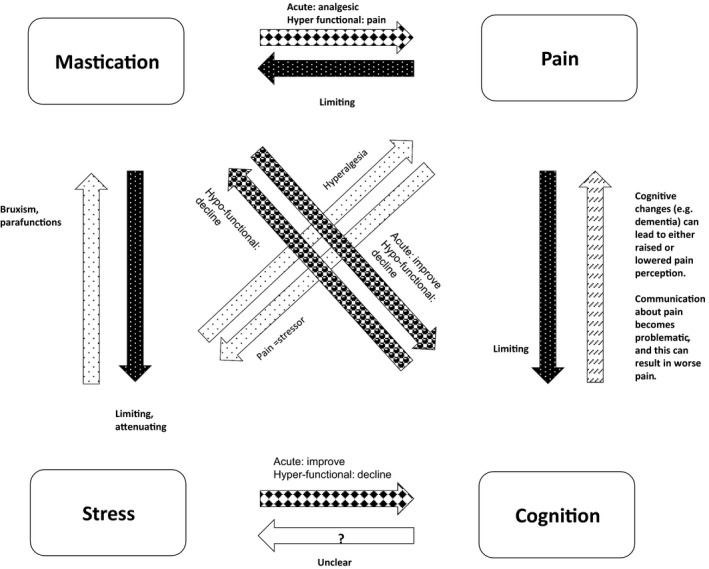
Interactions between mastication, cognition, stress and pain. Mastication has a regulatory effect on stress and also has positive effects on cognition and pain perception.12 In animals, reduced masticatory activity is linked to cognitive decline.5 Excessive mastication, however, can also cause pain.17 Pain causes stress32 and has a negative influence on masticatory activity,33 and on cognition.34 Cognitive changes such as dementia can both alter pain perception and limit pain communication.33 Cognitive decline can also have a negative effect on oral health.33 Research has not yet clearly revealed the nature of the influence that cognition may have on stress.35 Stress can lead to bruxism and/or oral parafunction12 and can also heighten sensitivity to pain (hyperalgesia).36 Although short‐term stress has a performance‐heightening effect, chronic stress has a negative effect on cognition12
5. CONCLUSION AND DISCUSSION
It could be plausible that a reciprocal relationship between mastication and cognition exists, as animal studies suggest, however, this relationship has not been sufficiently studied in human populations. It is probable that several factors play a role in this relationship, including self‐care, motor function, nutrition, stress and pain. A possible causal relationship between mastication and cognition is naturally of interest to anyone wanting to retain their cognitive strength, but it is perhaps of greatest importance to the group of vulnerable older people with dementia. It is therefore of great concern that oral health is particularly poor in this group.30
When considering functional recovery in older people with dementia, account must of course be taken of the feasibility and patient burden of treatment and the possibility of a reduced acceptance of prosthetics. Treatment interventions in this group should concentrate on prevention, comfort, dignity and pain control.31
ACKNOWLEDGEMENTS
This paper is an adapted and translated version of the Dutch article: Weijenberg, R.A.F., Delwel, S; Ho, BV; Wierink, CD; Lobbezoo, F. (2017) Denk aan je tanden‐ de relatie tussen kauwen en cognitie. Ned Tijdschr Tandheelkd, 2017. 124(9): p. 435‐440. https://doi.org/10.5177/ntvt.2017.09.16233; With permission from the Nederlands Tijdschrift voor Tandheelkunde B.V., Amsterdam, The Netherlands.
Weijenberg RAF, Delwel S, Ho BV, van der Maarel‐Wierink CD, Lobbezoo F. Mind your teeth—The relationship between mastication and cognition. Gerodontology. 2019;36:2–7. 10.1111/ger.12380
REFERENCES
- 1. Delwel S, Binnekade TT, Perez RS, Hertogh CM, Scherder EJ, Lobbezoo F. Oral health and orofacial pain in older people with dementia: a systematic review with focus on dental hard tissues. Clin Oral Invest. 2017;21(1):17‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zenthofer A, Baumgart D, Cabrera T, et al. Poor dental hygiene and periodontal health in nursing home residents with dementia: an observational study. Odontology. 2017;105(2):208‐213. [DOI] [PubMed] [Google Scholar]
- 3. Syrjala AM, Ylostalo P, Sulkava R, Knuuttila M. Relationship between cognitive impairment and oral health: results of the health 2000 health examination survey in finland. Acta Odontol Scand. 2007;65(2):103‐108. [DOI] [PubMed] [Google Scholar]
- 4. Watanabe Y, Hirano H, Matsushita K. How masticatory function and periodontal disease relate to senile dementia. Jpn Dent Sci Rev. 2015;51(1):34‐40. [Google Scholar]
- 5. Weijenberg RA, Scherder EJ, Lobbezoo F. Mastication for the mind‐the relationship between mastication and cognition in ageing and dementia. Neurosci Biobehav Rev. 2011;35(3):483‐497. [DOI] [PubMed] [Google Scholar]
- 6. Mendes Fde C, De Almeida MN, Felicio AP, et al. Enriched environment and masticatory activity rehabilitation recover spatial memory decline in aged mice. BMC Neurosci. 2013;14:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kondo H, Kurahashi M, Mori D, et al. Hippocampus‐dependent spatial memory impairment due to molar tooth loss is ameliorated by an enriched environment. Arch Oral Biol. 2016;61:1‐7. [DOI] [PubMed] [Google Scholar]
- 8. Huttenrauch M, Brauss A, Kurdakova A, et al. Physical activity delays hippocampal neurodegeneration and rescues memory deficits in an Alzheimer disease mouse model. Transl Psychiat. 2016;6:e800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weijenberg RA, Scherder EJ, Lobbezoo F. Mastication for the mind–the relationship between mastication and cognition in ageing and dementia. Neurosci Biobehav Rev. 2011;35(3):483‐497. [DOI] [PubMed] [Google Scholar]
- 10. Hasegawa Y, Ono T, Hori K, Nokubi T. Influence of human jaw movement on cerebral blood flow. J Dent Res. 2007;86(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe K, Ozono S, Nishiyama K, et al. The molarless condition in aged samp8 mice attenuates hippocampal fos induction linked to water maze performance. Behav Brain Res. 2002;128(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 12. Weijenberg RA, Lobbezoo F. Chew the pain away: oral habits to cope with pain and stress and to stimulate cognition. Biomed Res Int. 2015;2015:149431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erbay FM, Aydin N, Sati‐Kirkan T. Chewing gum may be an effective complementary therapy in patients with mild to moderate depression. Appetite. 2013;65:31‐34. [DOI] [PubMed] [Google Scholar]
- 14. Smith A. Effects of chewing gum on cognitive function, mood and physiology in stressed and non‐stressed volunteers. Nutr Neurosci. 2010;13(1):7‐16. [DOI] [PubMed] [Google Scholar]
- 15. Tucha L, Simpson W, Evans L, et al. Detrimental effects of gum chewing on vigilance in children with attention deficit hyperactivity disorder. Appetite. 2010;55(3):679‐684. [DOI] [PubMed] [Google Scholar]
- 16. Onozuka M, Fujita M, Watanabe K, et al. Age‐related changes in brain regional activity during chewing: a functional magnetic resonance imaging study. J Dent Res. 2003;82(8):657‐660. [DOI] [PubMed] [Google Scholar]
- 17. Farella M, Bakke M, Michelotti A, Martina R. Effects of prolonged gum chewing on pain and fatigue in human jaw muscles. Eur J Oral Sci. 2001;109(2):81‐85. [DOI] [PubMed] [Google Scholar]
- 18. Lexomboon D, Trulsson M, Wardh I, Parker MG. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J Am Geriatr Soc. 2012;60(10):1951‐1956. [DOI] [PubMed] [Google Scholar]
- 19. Scherder E, Posthuma W, Bakker T, Vuijk PJ, Lobbezoo F. Functional status of masticatory system, executive function and episodic memory in older persons. J Oral Rehabil. 2008;35(5):324‐336. [DOI] [PubMed] [Google Scholar]
- 20. Dworkin SF, Leresche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6(4):301‐355. [PubMed] [Google Scholar]
- 21. Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev. 2007;17(3):213‐233. [DOI] [PubMed] [Google Scholar]
- 22. Boelen D, Fasotti L, Spikman J. Aandacht en executieve functies In: Kessels R, ed. Klinische Neuropsychologie. Amsterdam, the Netherlands: Uitgeverij Boom; 2013:245‐265. [Google Scholar]
- 23. Hirano Y, Obata T, Kashikura K, et al. Effects of chewing in working memory processing. Neurosci Lett. 2008;436(2):189‐192. [DOI] [PubMed] [Google Scholar]
- 24. De Cicco V, Barresi M, Tramonti Fantozzi MP, Cataldo E, Parisi V, Manzoni D. Oral implant‐prostheses: new teeth for a brighter brain. PLoS One. 2016;11(2):e0148715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mcgarrigle R, Dawes P, Stewart AJ, Kuchinsky SE, Munro KJ. Pupillometry reveals changes in physiological arousal during a sustained listening task. Psychophysiology. 2017;54(2):193‐203. [DOI] [PubMed] [Google Scholar]
- 26. Ribeiro GR, Costa JL, Ambrosano GM, Garcia RC. Oral health of the elderly with Alzheimer's disease. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(3):338‐343. [DOI] [PubMed] [Google Scholar]
- 27. Elsig F, Schimmel M, Duvernay E, et al. Tooth loss, chewing efficiency and cognitive impairment in geriatric patients. Gerodontology. 2013;32(2):149‐156. [DOI] [PubMed] [Google Scholar]
- 28. Weijenberg RA, Lobbezoo F, Visscher CM, Scherder EJ. Oral mixing ability and cognition in elderly persons with dementia: a cross‐sectional study. J Oral Rehabil. 2015;42(7):481‐486. [DOI] [PubMed] [Google Scholar]
- 29. Miyake S, Yoshikawa G, Yamada K, et al. Chewing ameliorates stress‐induced suppression of spatial memory by increasing glucocorticoid receptor expression in the hippocampus. Brain Res. 2012;1446:34‐39. [DOI] [PubMed] [Google Scholar]
- 30. Syrjala AM, Ylostalo P, Ruoppi P, et al. Dementia and oral health among subjects aged 75 years or older. Gerodontology. 2012;29(1):36‐42. [DOI] [PubMed] [Google Scholar]
- 31. Volicer L, Simard J. Palliative care and quality of life for people with dementia: medical and psychosocial interventions. Int Psychogeriatr. 2015;27(10):1623‐1634. [DOI] [PubMed] [Google Scholar]
- 32. Sturgeon JA. Psychological therapies for the management of chronic pain. Psychol Res Behav Manag. 2014;7:115‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lobbezoo F, Weijenberg RA, Scherder EJ. Topical review: orofacial pain in dementia patients. A diagnostic challenge. J Orofac Pain. 2011;25(1):6‐14. [PubMed] [Google Scholar]
- 34. Scherder E, Eggermont L, Achterberg W, et al. [Pain and physical (in)activity in relation to cognition and behaviour in dementia]. Tijdschr Gerontol Geriatr. 2009;40(6):270‐278. [DOI] [PubMed] [Google Scholar]
- 35. King A. Neurobiology: rise of resilience. Nature. 2016;531(7592):S18‐S19. [DOI] [PubMed] [Google Scholar]
- 36. Alvarez P, Green PG, Levine JD. Stress in the adult rat exacerbates muscle pain induced by early‐life stress. Biol Psychiat. 2013;74(9):688‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]


