Figure 1. Structure and Biochemistry of Wild-type RAC1, the Constitutively Active RAC1Q61L mutant, and the Fast Cycling RAC1P29S Mutant.
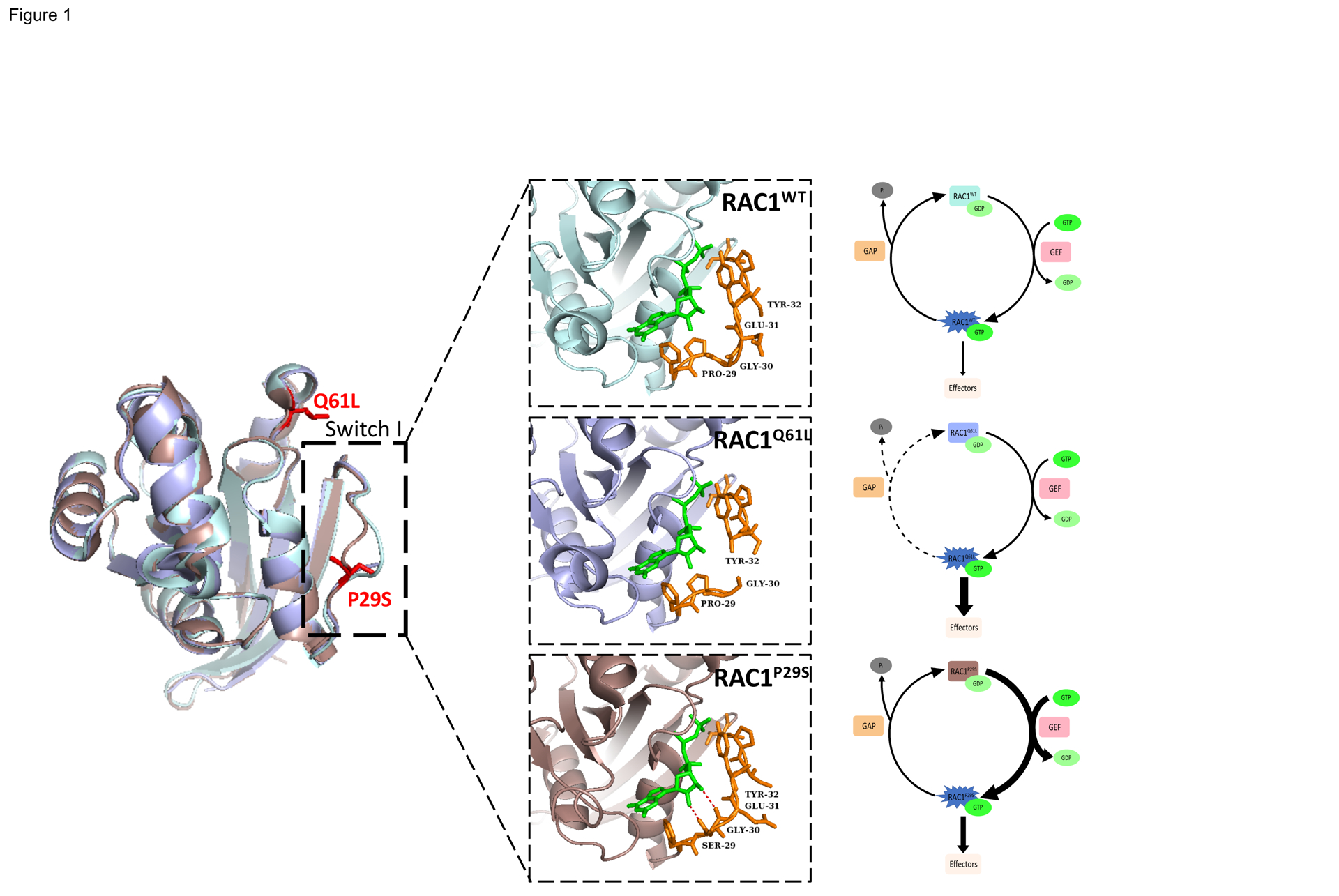
Comparison of crystal structures of RAC1WT (light blue, PDB 3TH5) with RAC1Q61L (light purple, PDB 4GZL), and RAC1P29S (dark pink, PDB 3SBE) in complex with the slow hydrolyzing GTP analog GMPPNP (green). RAC1P29S possesses a more flexible switch I domain than RACWT and RAC1Q61L. The proline to serine substitution enables hydrogen bonding between Ser-29 and Gly-30 of switch I and GTP and constitutes the difference between fast cycling mutations such as P29S and GTP-hydrolysis deficient mutations such as Q61L. Residue 29 and surrounding residues are displayed in orange stick format to illustrate hydrogen bonding (red).
