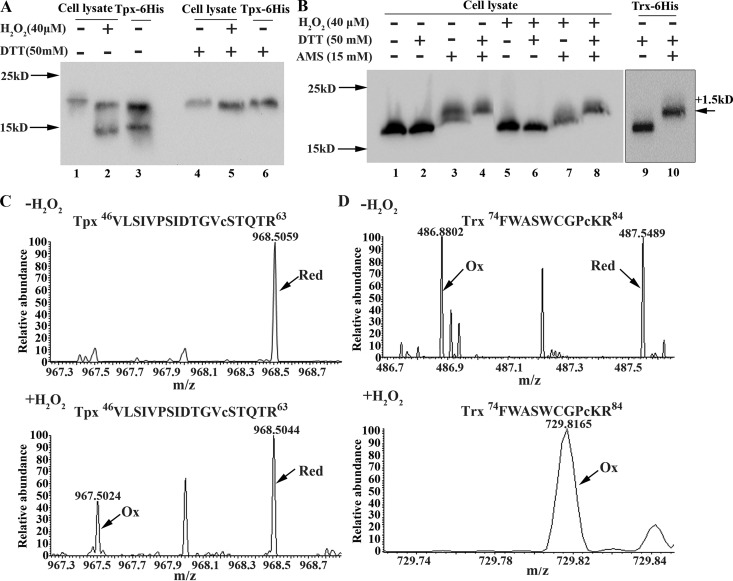
FIG 3.
Verification of the cysteine oxidation of thiol peroxidase (Tpx) and thioredoxin (Trx) in 40 μM H2O2-treated anaerobic cultures. (A) A 6×His tag was fused to the C terminus of the tpx gene (KEGG accession number I872_09640) to construct the S. oligofermentans Tpx-6×His strain. Mid-exponential-phase anaerobically grown Tpx-6×His cells were treated with or without 40 μM H2O2 for 20 min, collected inside an anaerobic glovebox, and then lysed in RIPA buffer containing the free thiol protectant NEM. The cell lysate of each sample was divided into two aliquots; one was left untreated (lanes 1 and 2), and the other was reduced with 50 mM DTT for 1 h (lanes 4 and 5). Redox Western blotting was carried out using an 18% SDS-PAGE gel to detect the Tpx-6×His protein using an anti-His tag antibody. Recombinant Tpx-6×His protein, which was partially oxidized and which formed an intramolecular disulfide linkage during purification, was treated with or without 50 mM DTT (lanes 3 and 6) and used as a reduced and an oxidized molecular control, respectively. (B) Using the same approach described in the legend to panel A, a disulfide linkage upon 40 μM H2O2 oxidation was identified for thioredoxin (KEGG accession number I872_03205) in the Trx-6×His strain (lanes 1 and 2 versus lanes 5 and 6). In addition, 15 mM 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS), the free thiol-chelating reagent, was used to detect the nondisulfide oxidation of the thiol groups (lanes 3 and 4 and lanes 7 and 8). Cell lysates from the H2O2-untreated strain (lanes 3 and 4) and the H2O2-treated Trx-6×His strain (lanes 7 and 8) were reduced with or without 50 mM DTT. The recombinant Trx-6×His protein was first reduced by 50 mM DTT, and then one aliquot was alkylated with AMS and another was left untreated; these were used as reduced and thiol AMS-bound Trx-6×His protein controls, respectively (lanes 9 and 10). Molecular weight markers are shown at the left, and the increased molecular weight of the protein due to bound AMS molecules (500 Da each) is shown at the right. (C and D) Redox proteomics identified the reduced (Red) and oxidized (Ox) peptide fragments of Tpx VLSIVPSIDTGVC58STQTR (C) and Trx FWASWCGPC82KR (D) in H2O2-untreated (top) and H2O2-treated S. oligofermentans cells (bottom). The relative abundances of the oxidized and reduced peptide fragments are shown.