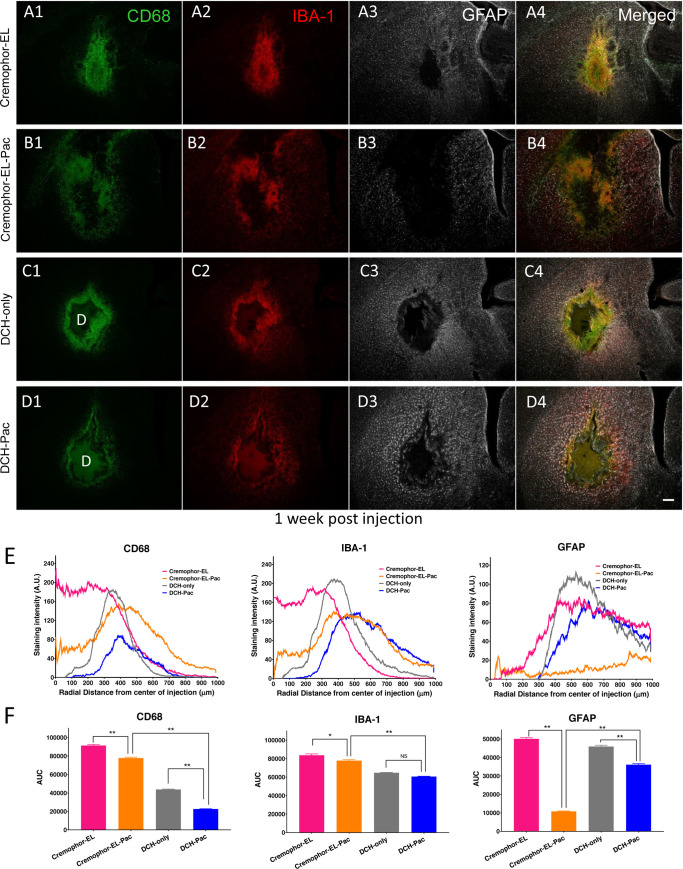
Fig 2. DCH-paclitaxel exhibits good biocompatibility in healthy CNS in contrast to paclitaxel in Cremophor EL vehicle.
A-D. Images of caudate putamen at 1 week after injection into healthy, uninjured tissue of Cremophor EL vehicle (A), Cremophor EL + paclitaxel (B), DCH only (C), or DCH + paclitaxel (D), showing single channel and merged multichannel immunofluorescence for multiple markers of inflammation and gliosis, CD 68, IBA-1 and GFAP. Scale bar, 200 μm for all images, D = DCH depot. E. Quantification of immunofluorescence intensity for each treatment group across a radial area of 1 mm originating from the center of the injection (n = 3 mice per stain per treatment). F. Area Under the Curve (AUC) calculations for the various immunofluorescence intensity traces from E. provide a single measure of cumulative staining within the 1 mm radial field. The DCH-paclitaxel system showed markedly and significantly less staining intensity, indicative of a more favorable foreign body response, compared with paclitaxel administered using the Cremophor EL vehicle * p < 0.01 or ** p < 0.001 (ANOVA with post-hoc Tukey’s multiple comparisons test).