Extended Data Fig. 3: Aβ levels in HSD and correlation of behavioral deficits with p-tau, as well as p-tau in hypertension, HSD-treated tg2576 mice and hypothermia,
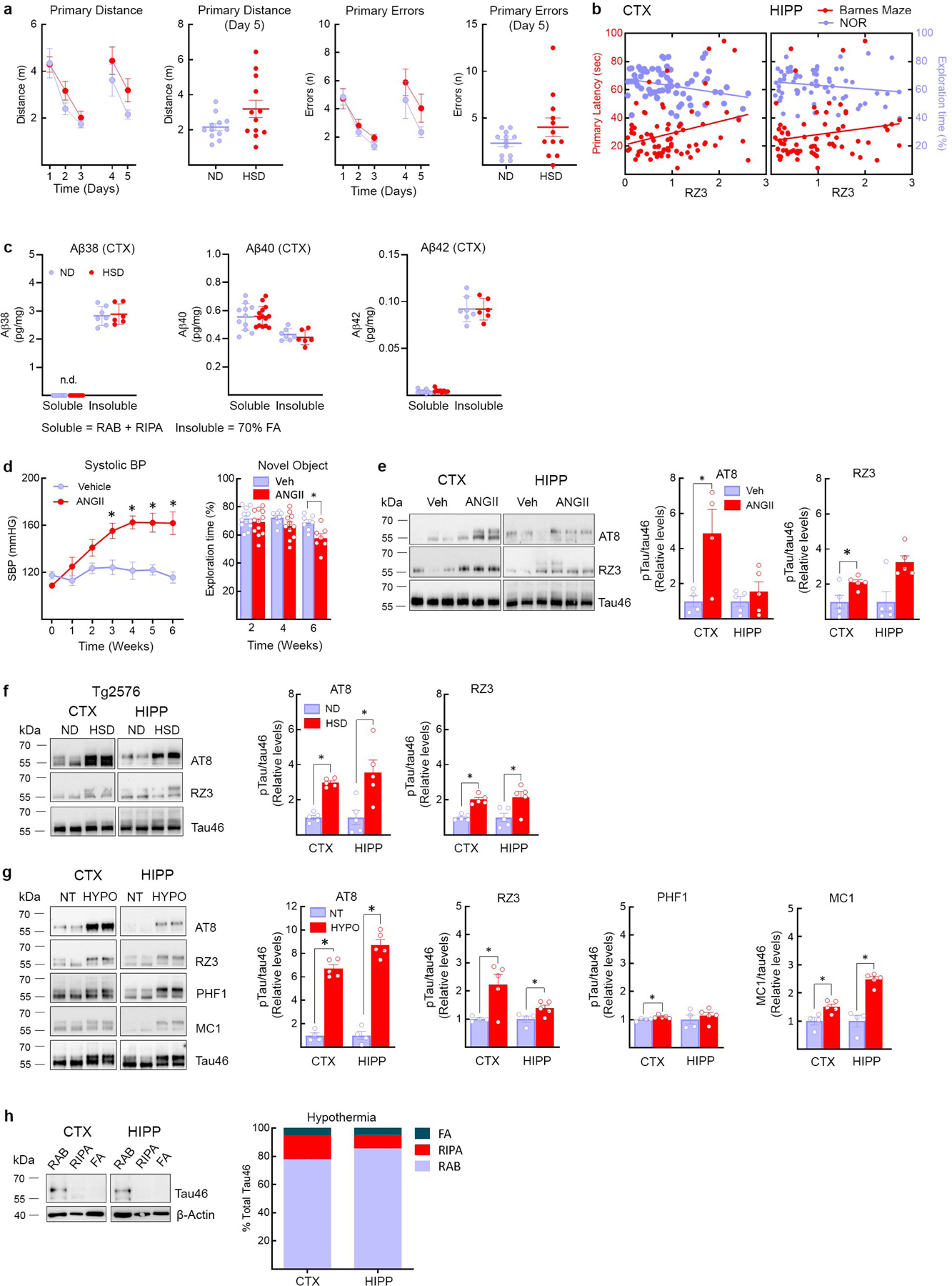
a,HSD (NaCl 8%) does not alter the distance travelled before finding the escape hole (Primary Distance, ND/HSD n=13/13, Diet: *p=0.0462, Time: *p<0.0001, two-way RM ANOVA plus Bonferroni’s test; Primary Distance Day 5: ND/HSD n=13/13, p=0.0670 vs ND, two-tailed unpaired t-test) or the number of errors made (Primary Errors, ND/HSD n=13/13, Diet: p=0.110, Time: *p=0.0004, two-way RM ANOVA plus Bonferroni’s test; Primary Errors, Day 5: p=0.1226 vs ND, two-tailed unpaired t-test). b, RZ3 levels in the cortex correlate with the cognitive performance at the novel object recognition test. No correlation was found between hippocampal RZ3 levels and cognitive performance at both the Barnes Maze and the novel object recognition test (RZ3 CTX: BM r=0.2828, *p=0.0133, n=76; NOR r=−0.2806, *p=0.0170, n=72; RZ3 HIPP: BM r=0.1739, p=0.1470, n=71; NOR r=−0.1746, p=0.1577, n=67, Pearson’s correlation coefficient). c: HSD did not increase soluble or insoluble Aβ38, Aβ40 or Aβ42 in neocortex (Aβ38, Soluble ND/HSD n=11/9, Insoluble ND/HSD n=7/6; Aβ40, Soluble ND/HSD n=11/14, Insoluble ND/HSD n=7/6; Aβ42, Soluble ND/HSD n=9/9, Insoluble ND/HSD n=7/6). d, Delivery of ANGII (600ng/kg·min, s.c.) with osmotic minipumps over 6 weeks increases systolic blood pressure and induces cognitive deficits (SBP – Veh/ANGII n=10/10, Treatment: *p<0.0001, Time: *p<0.0001, repeated two-way ANOVA and Bonferroni’s test; NOR – 2 weeks Veh/ANGII n=12/12, 4 weeks Veh/ANGII n=10/11, 6 weeks Veh/ANGII n=7/7, Treatment: *p<0.0021, Time: *p=0.0208, two-way ANOVA and Bonferroni’s test), e, ANGII administration increases AT8 and RZ3 in neocortex but not hippocampus (CTX, AT8 6 weeks: Veh/ANGII n=4/4, *p=0.0324 vs Veh; RZ3 6 weeks: Veh/ANGII n=5/5, *p=0.0262 vs Veh; HIPP, AT8 6 weeks: Veh/ANGII n=5/5, p=0.4056; HIPP, RZ3 6 weeks: Veh/ANGII n=5/5, p=0.0556, two-tailed unpaired t-test). f, HSD increases AT8 and RZ3 levels in both the neocortex and the hippocampus of 6 months-old Tg2576 mice (CTX, AT8: *p<0.0001 vs ND; HIPP, AT8: *p=0.0153 vs ND; CTX, RZ3: *p<0.0001 vs ND; HIPP, RZ3: *p=0.0239 vs ND, two-tailed unpaired t-test). g, Hypothermia induces massive AT8 phosphorylation (CTX: AT8 n=4/5, *p=0.0159 vs NT; HIPP: AT8 n=4/5, *p=0.0159 vs NT, unpaired two-tailed t-test). MC1 (CTX: MC1 n=4/5, *p=0.0317 vs NT; HIPP: MC1 n=4/5, *p=0.0159 vs NT, unpaired two-tailed t-test). RZ3 levels are also increased (CTX: RZ3 n=4/5, *p=0.0201 vs NT; HIPP: RZ3 n=4/5, *p=0.0453 vs NT, two-tailed unpaired t-test). h, At variance with HSD (Fig. 1G), hypothermia does not shift tau from soluble to more insoluble fractions. For gel source data see supplementary figure 1. Data are expressed as mean±SEM.
