Extended Data Fig. 5: GSK3β, Pin-1, calpastatin and Cdk5 nitrosylation in HSD, as well as neurovascular coupling, effect of HJ8.8 on p-tau, serum IL-17, and summary diagram.
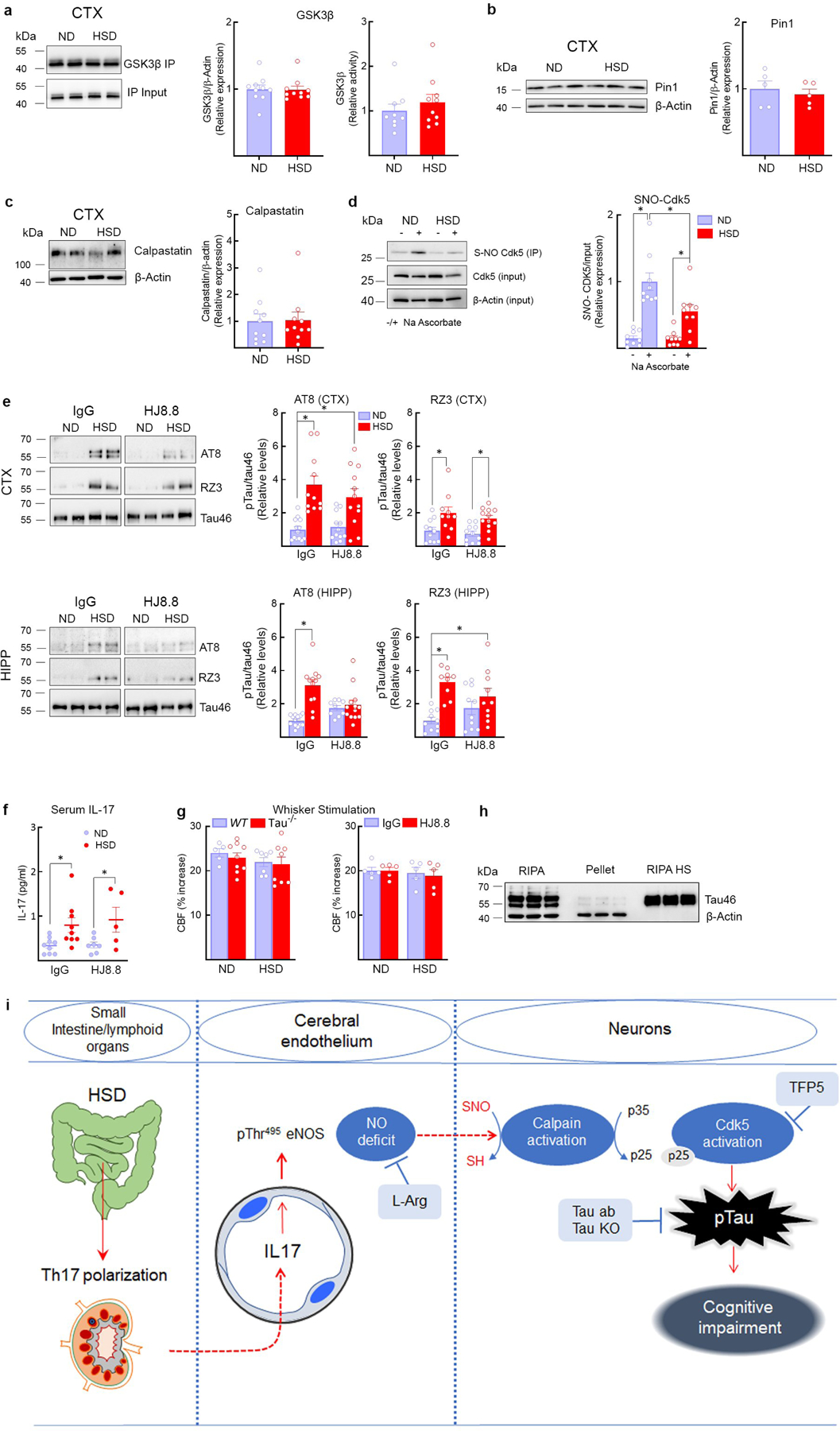
a, HSD has no effect on the expression and the activity of GSK3β in neocortex, ND/HSD n=10/10. b, HSD did not alter the expression of the prolyl cis/trans isomerase Pin-1, a regulator of tau dephosphorylation, ND/HSD n=5/5. c, The expression of calpastatin, an endogenous inhibitor of calpain activity, is not reduced by HSD, ND/HSD n=10/10. d, Nitrosylation of Cdk5 is reduced in the neocortex of HSD mice (ND/HSD n=9/9, Diet: *p=0.0143; Ascorbate: *p<0.0001, two-way ANOVA and Tukey’s test). e, HJ8.8 reduces AT8 levels in the hippocampus (AT8 – HIPP - IgG: ND/HSD n=13/12; HJ8.8: ND/HSD n=9/13; *p<0.0001, Kruskal-Wallis test and Dunn’s test). RZ3 levels are not altered by HJ8.8. f, Administration of HJ8.8 antibody does alter the increase of IL-17 serum levels induced by HSD (IgG: ND/HSD n=9/9, *p=0.0192 vs ND IgG; HJ8.8: ND/HSD n=7/5, *p=0.0421 vs ND HJ8.8, two-tailed unpaired t-test). g, The CBF increase in somatosensory cortex induced by neural activity evoked by mechanical stimulation of the whiskers is not reduced by HSD in WT, tau−/− and mice treated with the anti-tau antibody HJ8.8, WT ND/HSD n=5/7, Tau−/− ND/HSD n=9/8; IgG ND/HSD n=5/5, HJ8.8 ND/HSD n=5/5. h, Western blotting showing enrichment of tau in boiled RIPA neocortical samples (heat stable fraction, HS). Notice that β-actin is lost during the boiling process. Representative images from n=3 experiments. i, Cartoon depicting the mechanisms by which HSD leads to tau phosphorylation and cognitive impairment. HSD elicits a Th17 response in the small intestine, which leads to an increase in circulating IL17. IL17, in turn suppresses endothelial NO production by inducing inhibitory phosphorylation of eNOS at Thr495. The NO deficit results in reduced calpain nitrosylation in neurons, increased calpain activity, p35 to p25 cleavage, activation of Cdk5, and tau phosphorylation, which is ultimately responsible for cognitive dysfunction. In support of this chain of events, rescuing the endothelial NO deficit with L-arginine (L-Arg), lack of tau in tau-null mice, treatment with Cdk5 peptide inhibitor TFP5 or antibodies directed against tau (Tau Ab) prevent the cognitive dysfunction. For gel source data see supplementary figure 1. Data are expressed as mean±SEM.
