Fig. 4. Side-chain steric packing at PL5’s symmetric helix-helix interface.
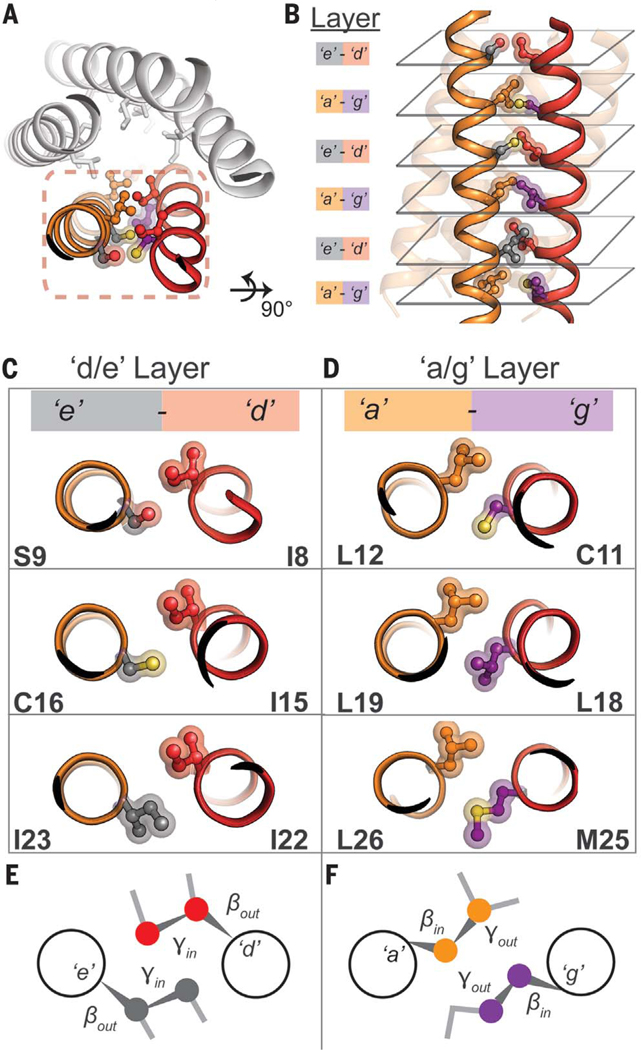
(A) The pairwise interaction of helices, symmetrically repeated, provides the primary stabilization for PL5. (B) High geometric complementarity of interacting residues across the helix-helix interface, roughly in layers: the a/g and e/g layers. (C and D) Axial view of side-chain packing of individual layers. (E) A potential stereochemical code required for pentameric assembly. At the e/d layer, the Cα-Cβ bond vector of each amino acid points outward from the helix-helix interface (βout), whereas the Cβ-Cγ bond vector faces inward (γin). This suggests that a heavy atom (e.g., N, C, O, S) at the gauche+ position is required for tight interhelical packing. (F) In the a/g layer, the opposite is true; the Cα-Cβ bond vector points inward (βin) and the Cβ-Cγ bond vector faces outward (γout).
