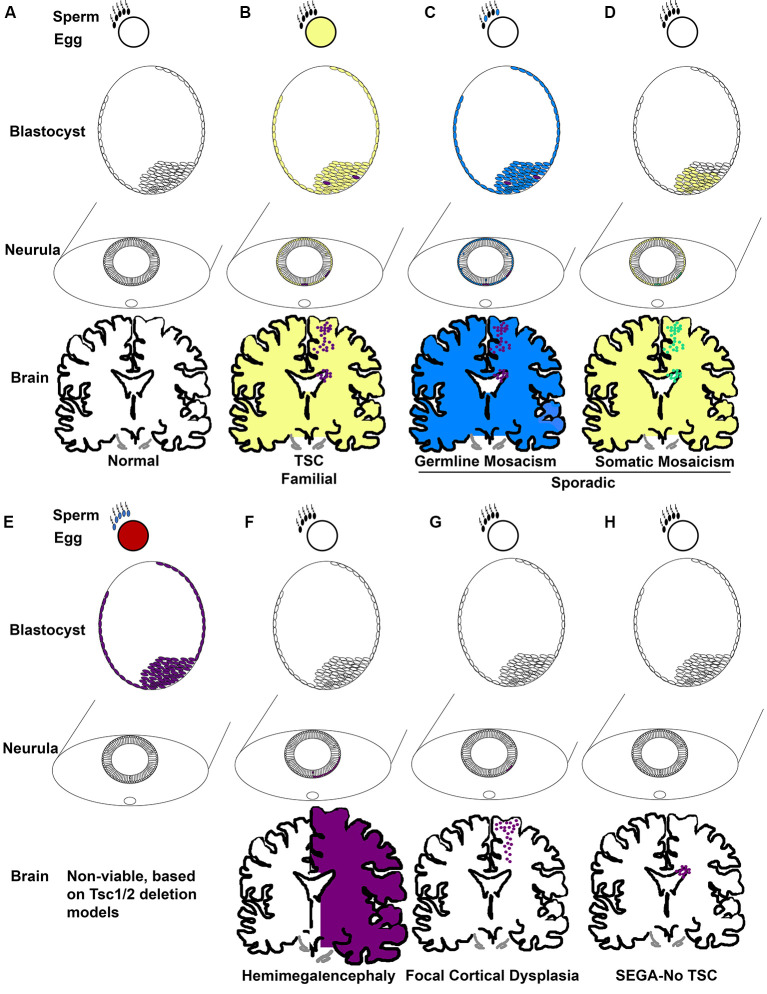
Figure 2.
TSC genetic mechanisms of pathogenesis. (A) Development commences when gametes with wild-type TSC alleles fuse. Development proceeds to the blastocyst phase with pluripotent cells of the inner cell mass becoming sequentially restricted in fate. Epiblasts of the blastocyst form the ectoderm which eventually undergoes primary neurulation and forms the neural tube. Neuroepithelial cells (NECs) that line the neural tube are stem cells that generate NSCs. NSCs, for instance, radial glia, generate neurons, astrocytes, oligodendrocytes, and ependyma. (B) Familial TSC arises when a haploid germ cell carries a mutation in TSC1 or TSC2 (yellow). A second mutation resulting in loss of heterozygosity (LOH; purple) then is thought to arise sporadically at different times of development, for example in the blastocyst in cells that eventually generate the CNS. (C) Germline mosaicism is phenotypically similar to familial TSC with the exception that only the parent’s germ cells might carry TSC gene mutations due to a sporadic mutation during gametogenesis (blue) followed by a second LOH mutation (purple). (D) Somatic mosaicism arises when a sporadic mutation occurs in one allele (yellow) during development, for example in the blastocyst, and LOH (green) occurs in subsets of cells later. (E) Animal models demonstrate that germ cells without a functional copy of a TSC gene (red, blue) produce embryos with no functional TSC gene (purple) that are non-viable at mid-gestation. (F) Hemimegalencephaly occurs when mutations (orange) in TSC genes arise and affect one side of the developing brain. (G) Focal cortical dysplasia occurs when TSC gene mutations (orange) arise in radial glia that generates cells within the cortical plate. (H) SEGA in the absence of TSC is predicted to occur when TSC gene mutations (orange) arise in a cell of the SVZ, potentially, NSCs.