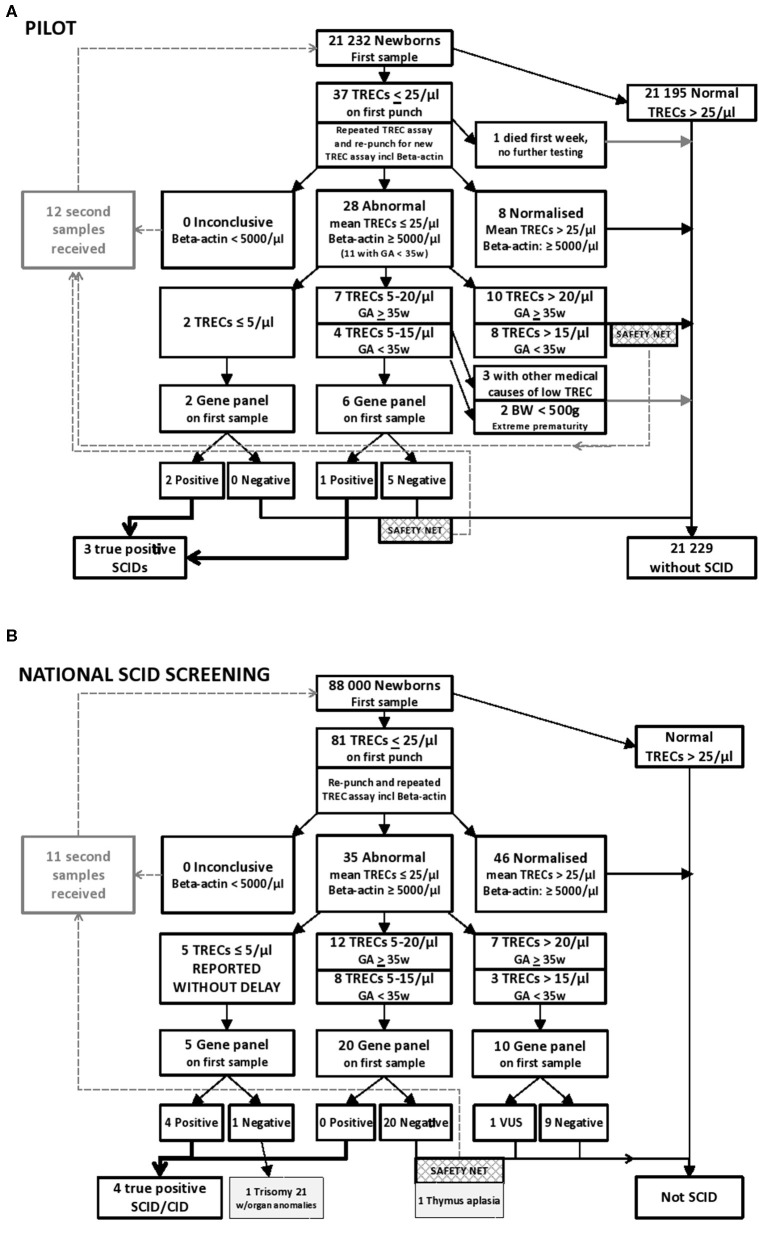
Figure 1.
(A) Results from the prospective pilot study with NGS gene panel testing integrated in the NBS laboratory algorithm for SCID and other T cell lymphopenias. Samples below 25 TRECs/μL were re-run and re-punched for one new TREC analysis. Samples with normal levels of β-actin (≥ 5,000/μL) and mean TREC levels below 20 TRECs/μL were NGS gene panel tested, dependent on gestational age (GA), birth weight (BW), and information in the baby's medical record. Out of the samples tested in the prospective pilot project, three individuals were identified with SCID. One of them had low intermediate TREC values, consistent with a “leaky” SCID. (B) Results from 20 months nationwide screening with rapid NGS gene panel testing integrated in the NBS laboratory algorithm for SCID and other T cell lymphopenias. Samples below 25 TRECs/μL were re-punched and TREC analyses repeated twice on DNA from the new punch. Samples with normal levels of β-actin (≥ 5,000/μL) and mean TREC levels below 25 TRECs/μL were NGS gene panel tested. Out of the samples tested in this nationwide screening between January 2018 to August 2019, 5 had TRECs below 5/μL, and four individuals were identified with severe primary immunodeficiency. The last one had Trisomy 21 with multiple anomalies. As a “safety net” a second DBS sample was requested if NGS was negative when TREC levels were below 20 TRECs/μL (15 TRECs/μL for prematures), which allowed for detection of one individual with congenital thymic aplasia.