Fig. 6. Intermittent treatment schedules with osimertinib and trametinib combination (A) delay the emergence of osimertinib resistance in vivo (B) without enhancing toxicity (C).
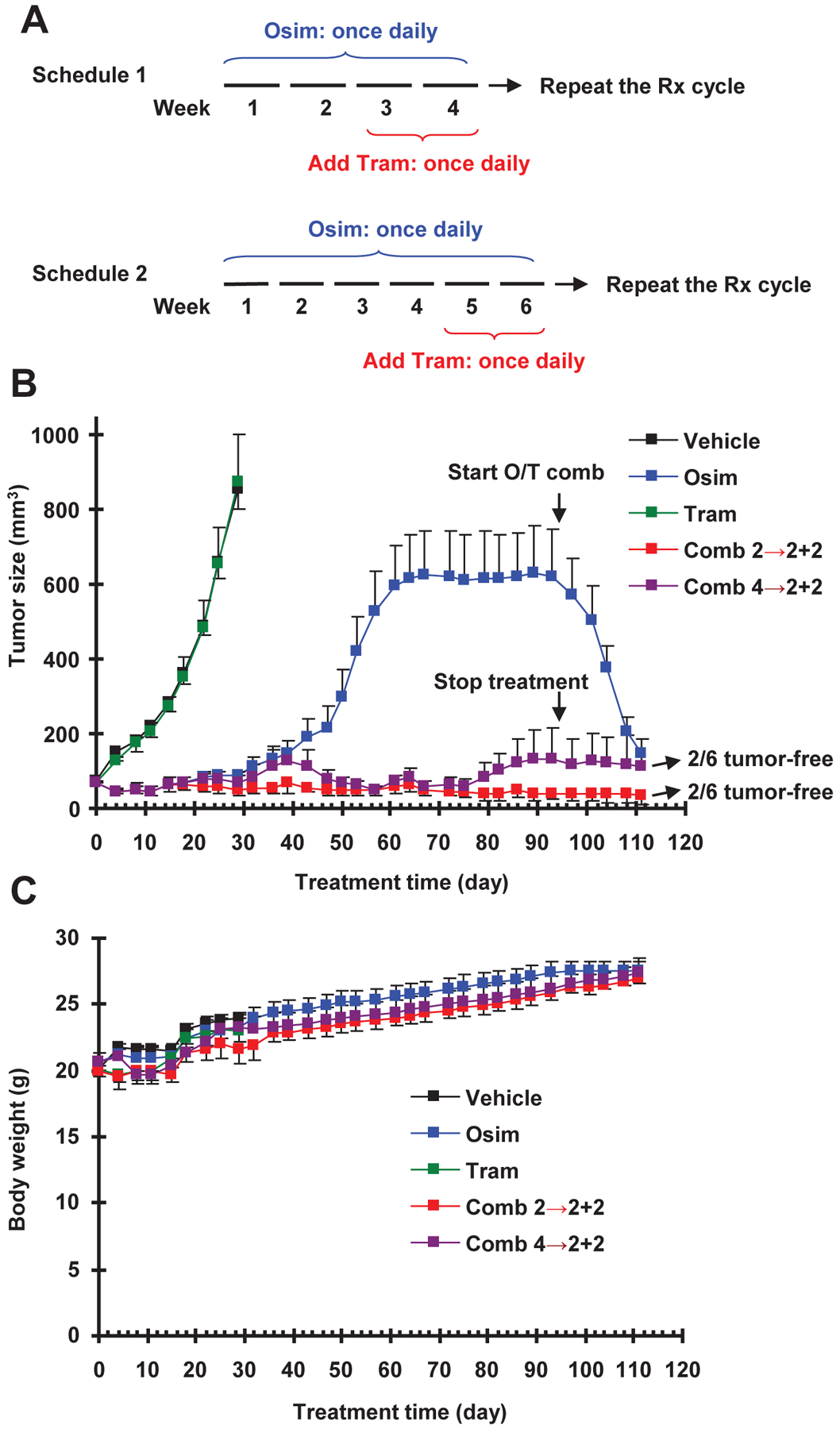
A, Schema of intermittent treatment schedules tested: Schedule #1 involved 2-weeks osimertinib treatment followed by 2-weeks osimertinib and trametinib combination and Schedule #2 used 4-weeks osimertinib treatment followed by 2-weeks osimertinib and trametinib combination. These schedules were repeated until the end of the experiments. B and C, PC-9 xenografts in nude mice (6 mice/group) were treated with vehicle, osimertinib (15 mg/kg, og, once/day), trametinib (1 mg/kg, og, once/day) or the combination of osimertinib and trametinib given according to Schedule 1 or Schedule 2. Tumor sizes and mouse body weights were measured every three days and presented as means ± SEMs.
