Figure 7. The human C-terminal pyrin B30.2 domain regulates pyrin inflammasome activation.
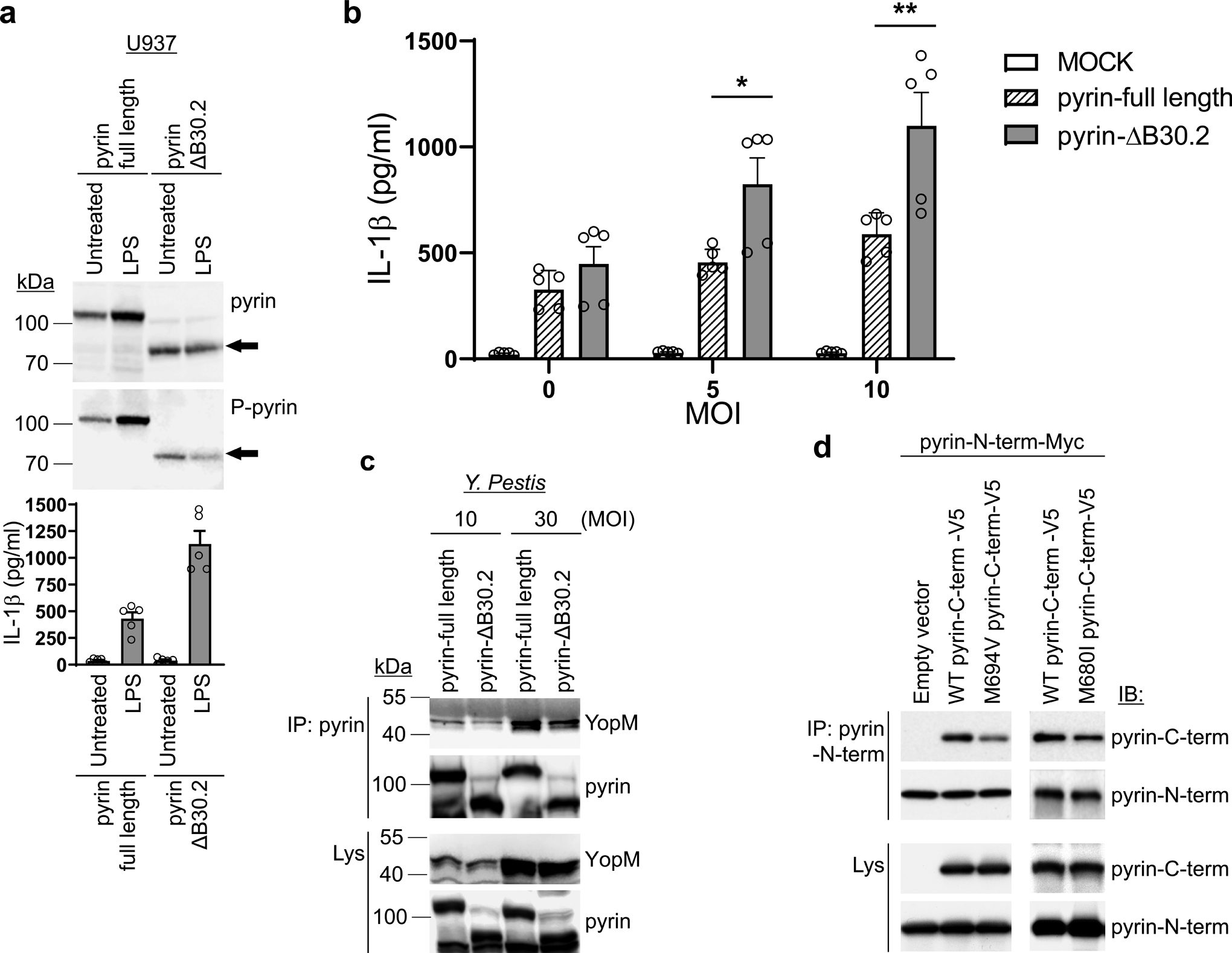
a. Immunoblot analysis of pyrin and phosphorylated pyrin in lysates of retroviral transduced U937 cells, expressing WT or B30.2 domain-deleted (ΔB30.2) pyrin, using antibodies specific for human pyrin and phosphorylated pyrin (top), and IL-1β measurements of culture supernatants with/without LPS treatment (bottom). Results are presented as mean ± s.e.m., for n=5 independent biological replicates. b. IL-1β measurements of culture supernatants from U937 cells expressing WT or B30.2 domain-deleted pyrin infected with indicated MOI of Y. pestis. Results are presented as mean ± s.e.m., for n=5 independent biological replicates. *P = 0.0317 and **P = 0.0079 (unpaired two-tailed t test). c. Immunoblot analysis of YopM in proteins immunoprecipitated with antibody to human pyrin (IP: pyrin) from lysates of U937 cells expressing WT or B30.2 domain-deleted pyrin infected with indicated MOI of Y. pestis. d. Immunoblot analysis of pyrin in proteins immunoprecipitated with antibody specific to N-terminal human pyrin (IP: Pyrin-N-term) from lysates of 293T cells expressing Myc- tagged N-terminal (amino acids 1–330) human pyrin with V5-tagged WT or mutant (p.M694V or p.M680I) C-terminal (amino acids 331–781) human pyrin. Data are representative of three independent experiments with similar results (a, c and d).
