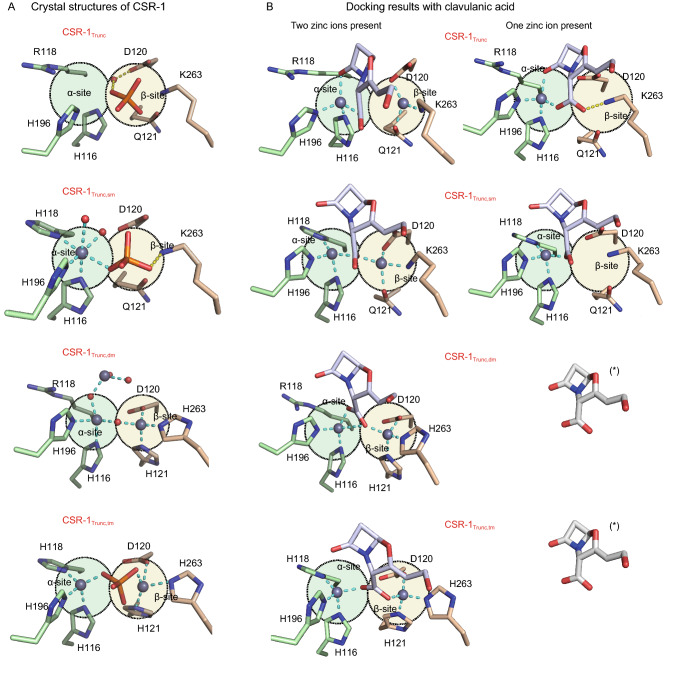
Figure 2.
Structural analysis of CSR-1 variants and their interaction with the inhibitor clavulanic acid. (A) Crystal structures of the active sites of the B3-RQK enzyme CSR-1 and its mutants highlighting the α- (green circle) and β- (yellow circle) metal binding sites. (B) Predicted docking of CA to CSR variants in the presence of one or two Zn2+ metal ions. The predicted binding of the bi-metallic forms of CSR-1 and its mutants are unlikely to represent the inhibited form of this enzyme because there is no difference in the four poses. In the presence of one metal ion, however, CA (*) is predicted to only form a stable enzyme-inhibitor complex with CSR-1 and its single mutant, consistent with the experimental inhibition data (Table S5)