Highlights
-
•
Cognitive impairment is a major comorbidity of temporal lobe epilepsy (TLE).
-
•
Three discrete cognitive phenotypes of TLE are identified here.
-
•
The phenotypes are linked to network, clinical, and socioeconomic characteristics.
-
•
This taxonomy advances clinical and theoretical understanding of the cognitive complications of TLE.
Keywords: Temporal lobe epilepsy (TLE), Phenotype, Cognitive impairment, Comorbidity, Socioeconomic status
Abbreviations: CI, Cognitive Impairment; ECP, Epilepsy Connectome Project; HCP, Human Connectome Project; ICV, Intra-Cranial Volume; rs-fMRI, Resting State Functional MRI; SES, Socio-Economic Status; TLE, Temporal Lobe Epilepsy
Abstract
This study explored the taxonomy of cognitive impairment within temporal lobe epilepsy and characterized the sociodemographic, clinical and neurobiological correlates of identified cognitive phenotypes. 111 temporal lobe epilepsy patients and 83 controls (mean ages 33 and 39, 57% and 61% female, respectively) from the Epilepsy Connectome Project underwent neuropsychological assessment, clinical interview, and high resolution 3T structural and resting-state functional MRI. A comprehensive neuropsychological test battery was reduced to core cognitive domains (language, memory, executive, visuospatial, motor speed) which were then subjected to cluster analysis. The resulting cognitive subgroups were compared in regard to sociodemographic and clinical epilepsy characteristics as well as variations in brain structure and functional connectivity. Three cognitive subgroups were identified (intact, language/memory/executive function impairment, generalized impairment) which differed significantly, in a systematic fashion, across multiple features. The generalized impairment group was characterized by an earlier age at medication initiation (P < 0.05), fewer patient (P < 0.001) and parental years of education (P < 0.05), greater racial diversity (P < 0.05), and greater number of lifetime generalized seizures (P < 0.001). The three groups also differed in an orderly manner across total intracranial (P < 0.001) and bilateral cerebellar cortex volumes (P < 0.01), and rate of bilateral hippocampal atrophy (P < 0.014), but minimally in regional measures of cortical volume or thickness. In contrast, large-scale patterns of cortical-subcortical covariance networks revealed significant differences across groups in global and local measures of community structure and distribution of hubs. Resting-state fMRI revealed stepwise anomalies as a function of cluster membership, with the most abnormal patterns of connectivity evident in the generalized impairment group and no significant differences from controls in the cognitively intact group. Overall, the distinct underlying cognitive phenotypes of temporal lobe epilepsy harbor systematic relationships with clinical, sociodemographic and neuroimaging correlates. Cognitive phenotype variations in patient and familial education and ethnicity, with linked variations in total intracranial volume, raise the question of an early and persisting socioeconomic-status related neurodevelopmental impact, with additional contributions of clinical epilepsy factors (e.g., lifetime generalized seizures). The neuroimaging features of cognitive phenotype membership are most notable for disrupted large scale cortical-subcortical networks and patterns of functional connectivity with bilateral hippocampal and cerebellar atrophy. The cognitive taxonomy of temporal lobe epilepsy appears influenced by features that reflect the combined influence of socioeconomic, neurodevelopmental and neurobiological risk factors.
1. Introduction
A longstanding pursuit in the neuropsychology of epilepsy has been an understanding of the signatures of cognitive abnormality associated with the disordered pathophysiology of specific epilepsy syndromes (Helmstaedter and Witt, 2012, Lin et al., 2012). This classic approach led to early appreciation of impaired memory in temporal lobe epilepsy (TLE), dysexecutive function in frontal lobe epilepsy, attentional disruption in absence epilepsy, language-related problems in Rolandic epilepsy, and dysexecutive behavior in juvenile myoclonic epilepsy (Elger et al., 2004, MacAllister and Schaffer, 2007). In addition, the association of cognitive abnormalities with clinical epilepsy characteristics (e.g., age of onset, seizure frequency/severity, duration of disorder) further clarified the presence, nature and severity of associated cognitive morbidity (Baxendale and Thompson, 2010, Dodrill and Matthews, 1992, Rudzinski and Meador, 2013). Early neuropathological and imaging studies contributed to an understanding of the links between syndrome-specific cognitive findings and anomalies in brain structure, a notable example being hippocampal pathology reflected in cell loss and gliosis and MRI-defined hippocampal atrophy linked with memory impairment in TLE (Lencz et al., 1992, Rausch, 1987, Sass et al., 1992, Sass et al., 1990, Trenerry et al., 1993). This general model, tracking cognition as a function of the taxonomy of the epilepsies and their associated clinical features, has served the field well (Loring, 2010, Novelly, 1992).
But some incongruities in the classic model have accumulated over the years, in part due to studies involving broad-based neuropsychological assessment comprehensively overviewing human cognition as well as by head-to-head cognitive comparisons of epilepsy syndromes. Rather than the expected selective cognitive abnormalities linked to syndrome-specific pathophysiology, either a) more widespread and arguably unexpected cognitive anomaly has been reported when epilepsy syndromes are studied in depth (e.g., generalized cognitive abnormalities in focal epilepsies) (Braakman et al., 2015, Guimaraes et al., 2007, Hwang et al., 2019, Marques et al., 2007, Oyegbile et al., 2004, Rzezak et al., 2007) or, b) in head-to-head comparisons of two or more epilepsy syndromes, considerably shared versus unique syndrome-specific cognitive abnormality is notable (Baxendale and Thompson, 2010, Braakman et al., 2015, Bremm et al., 2019, Guimaraes et al., 2007, Hwang et al., 2019, Jackson et al., 2013, Marques et al., 2007, Oyegbile et al., 2004, Rzezak et al., 2007, Smith, 2016, Wang et al., 2011), or c) particular cognitive impairments (e.g., dysexecutive function) have been found to cut across multiple epilepsy syndromes (Conant et al., 2010, Neri et al., 2012, Stretton and Thompson, 2012, Verche et al., 2018, Verrotti et al., 2015, Wandschneider et al., 2012).
These empirical findings, summarized in narrative, systematic and meta-analytic reviews (Fonseca Wald et al., 2019, Loughman et al., 2014, Nickels et al., 2016, Smith, 2016, Wickens et al., 2017, Wilson and Baxendale, 2014), appear complementary to modern neuroimaging studies detecting more extended than anticipated abnormalities in brain structure and connectivity within several epilepsy syndromes (Keller et al., 2015, Lin et al., 2007, McDonald et al., 2008, Nuyts et al., 2017, Otte et al., 2012, Slinger et al., 2016, van Diessen et al., 2014, Whelan et al., 2018), contributing to the contemporary perspective that neuropsychological abnormalities result from disruption in widely distributed cognition-dependent neuronal circuitry (Rayner and Tailby, 2017, Rayner et al., 2019, Wilson and Baxendale, 2014).
Moreover, no perspective seems able to adequately account for the individual variability inherent in cognition and associated underlying neurobiology within any epilepsy syndrome. An alternative paradigm is that within any epilepsy syndrome, and perhaps across epilepsy syndromes, there are so-called latent groups or cognitive phenotypes that exhibit distinct patterns of cognitive and perhaps associated neurobiological abnormality. In this view cognitive variability within an epilepsy syndrome becomes a core focus of investigation. A developing literature has demonstrated the existence of cognitive subtypes within well-characterized epilepsy syndromes, examined most frequently in TLE given the careful study of these patients as part of the presurgical process (Elverman et al., 2019, Hermann et al., 2007, Kaestner et al., 2019, Reyes et al., 2019, Rodríguez-Cruces et al., 2018). While the overall modal cognitive profile may be one of broader than expected abnormalities, subgroups of patients appear to exist including some with surprisingly intact cognition, some with syndrome-specific cognitive impairment, and others with generalized nonspecific cognitive impairment. The number of investigations examining neuroimaging correlates of these subgroups is more limited, but with some evidence that increasingly distributed neurobiological abnormalities are associated with increasingly broad cognitive impairments (Dabbs et al., 2009, Hermann et al., 2007, Kaestner et al., 2019, Reyes et al., 2019, Rodríguez-Cruces et al., 2018, Rodríguez-Cruces et al., 2020).
Here we continue to address the overarching hypothesis that cognition in epilepsy exhibits a taxonomy that only partially overlaps with syndrome-specific expectations. Our first aim is to identify the underlying cognitive phenotypes in a large group of participants with TLE from the Epilepsy Connectome Project (ECP) (Cook et al., 2019, Hwang et al., 2019). These patients are more representative of the general population of TLE patients as only a subset are medication-resistant and in need of surgical evaluation or eventual surgery. The second aim is to identify the network disruptions associated with these phenotypes, focusing on patterns of abnormality in resting-state functional MRI (rs-fMRI) as well as regional and network-based analyses of cortical-subcortical covariance in structural MRI. The final aim is to characterize the relationship of identified cognitive phenotypes to relevant clinical and sociodemographic predictors—including factors reflective of socioeconomic status which are under-investigated in this literature.
2. Materials and methods
2.1. Participants
Participants included 111 TLE patients and 83 healthy control volunteers prospectively enrolled from the ECP (See Table 1) (Cook et al., 2019, Hwang et al., 2019). ECP is a two-site research project involving the Medical College of Wisconsin and the University of Wisconsin-Madison, reviewed and approved by the IRB (Institutional Review Board) at the Medical School of Wisconsin and all participants provided written informed consent, all procedures consistent with the Declaration of Helsinki.
Table 1.
Participants. Controls and TLE characteristics (2nd and 3rd columns) and the three cognitive phenotype groups (4th through 6th columns). Generalized Cognitive Impairment (Generalized-CI), Focal Cognitive Impairment (Focal-CI) and No Cognitive Impairment (No-CI) – refer to TLE patient subgroups identified through the clustering analysis in Section 2.3.1. †Based on adjusted hippocampal z-score of <−1.5. If based on z < −1.0, 43% of TLE exhibited hippocampal atrophy. ‡One value missing from Generalized-CI. Please see text for interpretation of significant differences. (last column).
| Groups | Controls | All TLE | Generalized-CI | Focal-CI | No-CI | p (ANOVA/χ2) |
|---|---|---|---|---|---|---|
| N | 83 | 111 | 20 | 34 | 57 | – |
| Age (years) [Mean ± SD] | 33.8 ± 10.6 | 39.6 ± 11.5 | 38.2 ± 13.5 | 36.6 ± 11.1 | 41.9 ± 10.4 | 0.17 |
| Gender (Male/Female) | 36/47 | 43/68 | 8/12 | 15/19 | 20/37 | 0.91 |
| Education (years) [Mean ± SD] | 15.8 ± 2.7 | 14.7 ± 2.7 | 12.3 ± 2.0 | 13.6 ± 1.7 | 16.2 ± 2.4 | <0.001 |
| Mother Education (year) [Mean ± SD] | 14.6 ± 2.7 | 13.5 ± 2.7 | 12.6 ± 2.7 | 13.8 ± 2.3 | 13.6 ± 2.9 | <0.05 |
| Father Education (years) [Mean ± SD] | 14.8 ± 2.8 | 13.8 ± 2.9 | 11.9 ± 2.1 | 13.2 ± 2.0 | 14.7 ± 3.1 | <0.001 |
| Race (Caucasian/Non-Caucasian) | 74/9 | 91/20 | 10/10 | 26/8 | 55/2 | <0.05 |
| Duration of Seizures (years) [Mean ± SD] | – | 16.8 ± 13.9 | 21.3 ± 16.7 | 13.2 ± 12.9 | 17.4 ± 13.2 | 0.17 |
| Recurring Seizure Onset Age (years) [Mean ± SD] | – | 22.8 ± 13.6 | 16.8 ± 11.9 | 23.4 ± 11.5 | 24.5 ± 14.6 | 0.17 |
| Drug Onset Age (years) [Mean ± SD] | – | 25.7 ± 13.6 | 18.3 ± 11.1 | 24.4 ± 11.2 | 29.0 ± 14.5 | <0.05 |
| Number of Anti-epileptic Drugs [Mean ± SD] | – | 1.8 ± 0.9 | 2.4 ± 0.7 | 1.8 ± 1.0 | 1.7 ± 0.9 | 0.07 |
| Seizure Laterality (Left/Right/Bilateral/Uncertain) | – | 57/25/9/20 | 9/6/2/3 | 17/9/2/6 | 31/10/5/11 | 0.91 |
| Seizure Controlled for >1 year (Yes/No) | – | 61/50 | 8/12 | 20/14 | 33/24 | 0.66 |
| Hippocampal Atrophy (HA) [unilateral/bilateral]† | 2.5% | 23% | 33% [7%/26%] | 23% [13%/10%] | 20% [11%/10%] | <0.05 |
| Generalized Seizure History (Yes/No) | – | 55/56 | 16/4 | 15/19 | 24/33 | <0.05 |
| Number of Lifetime Generalized Seizures (0–5/6–50/51+)‡ | – | 61/40/9 | 3/11/5 | 19/12/3 | 39/17/1 | <0.001 |
Eligible TLE patients were between the ages of 18 and 60, had tested full-scale IQ (Intelligence Quotient) at or above 70, spoke English fluently, with no medical contraindications to MRI. The diagnosis of TLE was supported by two or more of the following: 1) described or observed clinical semiology consistent with seizures of temporal lobe origin, 2) EEG evidence of either Temporal Intermittent Rhythmic Delta Activity or temporal lobe epileptiform discharges, 3) temporal lobe onset of seizures captured on video EEG monitoring, or 4) MRI evidence of mesial temporal sclerosis or hippocampal atrophy. Patients with any of the following were excluded: 1) lesions other than mesial temporal sclerosis causative for seizures, and 2) an active infectious/autoimmune/inflammatory etiology of seizures.
The TLE cohort is not a surgical sample with a modest proportion (34%) of participants who underwent ictal monitoring. This in turn has limitations, primarily in regard to unequivocal seizure lateralization. However, the proportion of patients who underwent ictal monitoring is quite similar to the prevalence of medication refractory epilepsy (Kwan and Brodie, 2000). In this regard the cohort is less biased toward medication refractory/surgical TLE and therefore more representative of TLE in general. Nonetheless we appreciate the inherent limitations in using seizure frequency reports provided by patients and extracted from medical records.
The controls were healthy adults between the ages of 18 and 60. Exclusion criteria included: Edinburgh Laterality (Handedness) Quotient less than +50; primary language other than English; history of any learning disability, brain injury or illness, substance abuse, or major psychiatric illness (major depression, bipolar disorder, or schizophrenia); current use of vasoactive medications; and medical contraindications to MRI.
The epilepsy and control groups differed in age (P < 0.05) but not gender distribution (P = 0.13). Age was controlled in all analyses to be described including cognition, graph theory, cortical thickness and volume, and rs-fMRI.
2.2. Data acquisition
2.2.1. Neuropsychological assessment
All control and TLE participants underwent neuropsychological evaluation targeting assessment of intelligence, language, visuoperceptual/constructional skills, learning and memory, executive functions, and cognitive/psychomotor speed. A total of 18 cognitive indices resulted, which included assessment of intelligence (Wechsler Abbreviated Scale of Intelligence-2 Vocabulary and Block Design subtests) (Wechsler, 2011), verbal learning and memory (Rey Auditory Verbal Learning Test) including total words learned across trials (Rey, 1964), object naming (Boston Naming Test) (Kaplan et al., 1983), letter fluency (Controlled Oral Word Association Test) (Heaton et al., 2004, Spreen and Benton, 1977), semantic fluency (Animal Naming) (Heaton et al., 2004, Strauss et al., 2006), spatial orientation (Judgement of Line Orientation) (Benton et al., 1983), face recognition (Facial Recognition Test) (Benton et al., 1983), speeded fine motor dexterity (Grooved Pegboard, dominant and non-dominant hands) (Klove, 1963), and selected subtests from the National Institutes of Health Toolbox-Cognitive Battery including the Pattern Comparison Processing Speed Dimensional Change Card Sort, List Sorting Working Memory, Flanker Inhibitory Control and Attention, Picture Vocabulary, Oral Reading Recognition, and Picture Sequence Memory tests (Carlozzi et al., 2014, Carlozzi et al., 2015).
2.2.2. Neuroimaging
Per protocol, 55 controls and all TLE patients underwent neuroimaging. MRI was performed on 3T General Electric (GE) 750 scanners at both institutions. T1-weighted structural images were acquired using MPRAGE (reduced magnetization prepared gradient echo sequence, TR/TE = 604 ms/2.516 ms, TI = 1060.0 ms, flip angle = 8°, FOV = 25.6 cm, voxel size = 0.8 mm isotropic). Four 5-minute rs-fMRI images acquired over two sessions using whole-brain simultaneous multi-slice, gradient echo planer imaging (Moeller et al., 2010) (8 bands, 72 slices, TR/TE = 802 ms/33.5 ms, flip angle = 50°, matrix = 104 × 104, FOV = 20.8 cm, voxel size = 2.0 mm isotropic) and a 32-channel receive coil were concatenated. The participants were asked to fixate on a white cross at the center of a black screen during the scans for better reliability (Patriat et al., 2013).
2.3. Data processing
2.3.1. Neuropsychological data
Each raw test score was regressed on age for the control data. Other demographic (e.g., gender, education) variables were of interest as predictors of cluster membership and were not used for norming purposes. Scores were normalized to z-scores with the mean and standard error of the control data used to compute z-scores for the patients. Regression assumptions were checked using both plots and statistical tests. No obvious patterning nor deviations from linearity were seen in a residual versus fitted value plot using the control data. Residual normality was investigated both visually using a quantile–quantile comparison plot as well as statistically using Shapiro-Wilks test.
The cognitive tests were then grouped by clinical consensus to five domains, and the internal consistency of each cognitive domain was subsequently evaluated by Cronbach’s alpha for the control group. Tests with relationships to more than one domain (e.g., verbal fluency tasks, which have both language and executive components) were placed in the domain with which they had the highest item-total correlation. The resulting domains were subsequently examined using Cronbach’s alpha in the TLE group, followed by exploratory factor analysis to demonstrate method invariance using principal axis factoring and Promax rotation with Kaiser normalization.
With regard to available empirical methods for determining numbers of factors to retain, unfortunately there is no method that has been found to be optimal under all circumstances. While many recommend parallel analysis, there have been concerns that it may be overly lenient if the mean eigenvalue is used (Glorfeld, 1995) but significantly underfactor at the 95th percentile in instances when factors are correlated and the number of indicators per factor are small (Crawford et al., 2010). Both of these conditions are present in the current study. For our study, parallel analysis suggests three factors when using the 95th percentile and six factors when using the mean. Another more recently proposed measure, the empirical Kaiser criterion (EKC), takes into account distributional considerations such as the size of the first factor and has been found to perform as well or better than parallel analysis in some cases in which factors are correlated and the number of variables per factor was small (Braeken and van Assen, 2017). When combining the EKC with the traditional rule, the result was a five-factor solution.
Composite scores were created for each domain using the mean z-score for the component measures, and these composite scores were then subjected to k-means clustering. The resulting clusters then served as the basis for examination of their correlates with sociodemographic and clinical seizure features and neuroimaging results.
2.3.2. MRI image preprocessing
MRI images were processed using the Human Connectome Project (HCP) minimal processing pipelines (Glasser et al., 2013) which are primarily based on FreeSurfer v5.3 (Dale et al., 1999) and FSL (Functional MRI of the brain Software Library) (Jenkinson et al., 2012). In brief, the function of this pipeline is to register T1-weighted images to the Montreal Neurological Institute (MNI) space, segment the volume into predefined structures, reconstruct white and pial cortical surfaces, and perform FreeSurfer's standard folding-based surface registration to a surface atlas. The functional portion of the pipelines removes spatial distortions in the rs-fMRI images using spin echo unwarping maps, realigns volumes to compensate for subject motion, registers to the structural images, reduces the bias field, normalizes the 4D image to a global mean, masks the data with the final brain mask and maps the voxels within the cortical gray matter ribbon onto the native cortical surface space. Details on the HCP processing pipelines can be found in Glasser et al. (2013).
254 structural features generated by FreeSurfer’s standard reconstruction (recon-all) were extracted from the T1-weighted images, including cortical thicknesses, surface areas, volumes and also subcortical and global volumes. Surface areas and volumes were divided by the total surface area and total gray matter volume respectively to normalize for brain size. Then the structural features were normalized through z-score transform.
Additional pre-processing was performed on the rs-fMRI images using AFNI (Analysis of Functional Neuro-Images) (Cox, 1996). This included motion regression using 12 motion parameters, regression-based removal of signal changes in the white matter, CSF, global signal, and band-pass filtering (0.01–0.1 Hz). There are ongoing efforts to determine the best motion correction method for multiband images (Hoinkiss et al., 2019, Williams and Van Snellenberg, 2019). Here, the combination of preprocessing pipelines recommended by the HCP, which included frame-wise registration to the single-band reference image to correct for head motion (S. M. Smith et al., 2013) and regressing 12 motion parameters (X/Y/Z, pitch/roll/yaw and their temporal derivatives), as well as signals from WM, CSF and global signal (Power et al., 2012) was considered adequate for our data. There were no differences in absolute or relative RMS motion between the clusters (P > 0.3).
Time-series data from four 5-minute rs-fMRI scans acquired in a single session were concatenated. 360 time-series from Glasser Parcellation (Glasser et al., 2016) plus 19 FreeSurfer subcortical regions (Fischl et al., 2002) were extracted per subject. Pairwise Pearson correlations between 379 time series (71,631 connections) were calculated and Fisher-z transformed for generating connectivity matrices.
2.3.3. Graph theory measures
Symmetric matrices of 87 nodes (descriptions in Supplementary Table 1) were calculated for the healthy controls and each TLE cluster group based on the Desikan‐Killiany probabilistic atlas. Matrices were calculated based on the partial correlations between node volumes controlling for intracranial volume (ICV). Subsequently, both global and local measures were calculated from thresholded matrices ranging from 15% to 35% density. This was calculated using a combination of proportional thresholding and minimum spanning tree (see Garcia-Ramos et al., 2015 for further methodological details). In short, the thresholding method used a combination of the minimum spanning tree of the graph plus a proportional threshold of the density of interest; we refer to these matrices as having a hybrid threshold. Global measures were acquired at different hybrid thresholds in order to ensure that results hold regardless of network density. Local measures were calculated at a hybrid threshold of 25%. The Force Atlas 2 algorithm of the open source software Gephi 9.2 (https://gephi.org) was used for the 2D visualization of modularity and community structures (scaling = 2000).
2.4. Comparisons between TLE clusters
2.4.1. Structural brain
Freesurfer's QDEC (Query, Design, Execute, Contrast) was used to perform surface-based analysis for cortical volume (age, gender, ICV covariates) and thickness (age and gender covariates) measurements. Each subject's native surface measures were mapped to the atlas surface of “fsaverage” to allow between-subject comparisons. Surface data were smoothed with a 10-mm FWHM (full-width-at-half-maximum) filter. To correct for multiple comparisons, a Monte-Carlo simulation was implemented with a cluster forming threshold set to P = 0.05. Clusters were tested against an empirical null distribution of maximum cluster size built using synthesized Z distributed data across 10,000 permutations, producing cluster wise P-values fully corrected for multiple comparisons. Also compared across groups was estimated ICV (adjusted for age and gender), total gray and white matter, cerebellum and hippocampus (adjusted for age, gender, and ICV), all processed using Freesurfer v5.3, using ANCOVA and MANCOVA.
2.4.2. Graph theory
The MATLAB-based Brain Connectivity Toolbox (http://www.brain-connectivity-toolbox.net/) was used to calculate graph theory measures. Weighted-symmetric adjacency matrices were created for each group based on the correlation coefficient of the volume covariance between each pair of nodes. Matrices were proportionally thresholded after the Minimum Spanning Tree (MST) was added as its backbone (see Garcia-Ramos et al., 2015, for details). To statistically investigate group differences, each group matrix was resampled by replacement (i.e., bootstrapped) a total of 500 times. Since results from graph theory measures can occur by chance, each graph measure was calculated on 500 random matrices with the same number of nodes and degree distribution as the pertinent graphs. In this way, the null hypothesis could be tested. P-values were corrected for multiple comparisons for each of the global and local measures. Specifically, Bonferroni correction for the global analyses was based on the standard alpha level of 0.05 divided by the number of tests (three in total) multiplied by the permuted matrices of two groups (5002) to the power of the number of thresholds used (11 in total). For the betweenness centrality analysis, Bonferroni correction was based on the standard alpha level of 0.05 divided by the number of nodes (87 in total) and multiplied by the permuted matrices of two groups (5002 in total). Graph theory measures were obtained from each resampled matrix at different hybrid thresholds (i.e., combination of MST and proportional threshold), and averages for the global measures were used for evaluations.
The global measures investigated included global efficiency, transitivity, community structure, and modularity index (Q), using modularity Louvain algorithm, and were calculated over a range of hybrid thresholds (0.15–0.35; 0.02 intervals). These measures have been thoroughly described in previous work (Garcia-Ramos et al., 2016). Global efficiency examines network integration (Wang et al., 2010). Transitivity characterizes the level of segregation of the network, i.e., the degree of clustering of nodes in the network. Finally, the community structure indexes the configuration of a network into segregated communities, while modularity index speaks to how easily the communities are identified by the algorithm. Given that the modularity algorithm provides a statistical estimate for each output (Blondel et al., 2008), we calculated modularity 1000 times for each group, and the highest proportion was chosen as the number of modules in that group. Betweenness centrality was calculated for each group at a hybrid threshold of 25%. The threshold of 25% was chosen by calculating an average across all four groups for each global measure in order to find a maximum (or minimum) value on the curve that results across thresholds. Although subtle, the only measure giving, in this case a maximum in the analysis was the transitivity graph, which resulted in a threshold of 25%. Betweenness centrality represents the relevance of a node for the communication between other nodes in the network (Boccaletti et al., 2006). Nodes with high betweenness centrality facilitate global integrative processes, serving as “highways” to ease “traffic” flow in the network (Sporns et al., 2007).
2.4.3. Resting state functional connectivity
Unique pairwise correlations in the connectivity matrices were compared between the healthy controls and each of the three clusters, using GLM (reduced generalized linear model), with chronological age as a covariate. P-values were corrected for multiple comparisons using the Benjamini-Hochberg false discovery rate correction based on the standard alpha level of 0.05.
2.5. Data availability
Efforts are ongoing to release raw DICOM (digital imaging and communications in medicine) data from the ECP through the CCF (Connectome Coordination Facility, www.humanconnectome.org/software/connectomedb) (Hodge et al., 2016) at Washington University in St. Louis.
2.6. Statistical analysis
Details regarding analyses of the cognitive and all imaging data (regional and network approaches, and resting-state) are specified in the pertinent sections.
3. Results
Table 1 provides an overview of the control and TLE participants as well as the cognitive phenotype groups across sociodemographic and clinical variables.
3.1. Neuropsychology
Final Cronbach’s alphas for the five cognitive domains in the control group suggested reasonably strong internal consistency: Language = 0.812 (N = 34), Executive Function/Processing Speed = 0.701 (N = 79), Memory = 0.847 (N = 35), Visuospatial = 0.695 (N = 31), and Motor Speed = 0.785 (N = 83). After creating composite scores for the patients with TLE based on these domains, one participant was an extreme outlier in the Motor Speed domain and was removed. The Cronbach’s alphas for the five domains in this group based on the remaining 111 participants with TLE also showed reasonably strong internal consistency: Language = 0.879, Executive Function/Processing Speed = 0.777, Memory = 0.774, Visuospatial = 0.683, and Motor = 0.862. Factor analysis yielded five factors with eigenvalues>1, which corresponded well to the five domains created based on theoretical justification and the Cronbach’s alpha results in the controls. All but two measures (Picture Sequence Memory and Pattern Comparison Processing Speed) showed substantial loadings (pattern coefficients > 0.45) on the factor corresponding to the domain it had been assigned, and all but one measure (Picture Sequence Memory) showed its highest loading on that domain. Consistent with published literature, all mean cognitive domain scores for the TLE group were significantly (P < 0.05) lower than controls across all domains (Sidak corrected).
The psychometric tests falling within each cognitive domain were as follows: Language (WASI Vocabulary, Boston Naming Test, ToolBox Picture Vocabulary, Oral Reading); Executive/processing speed (Letter/Category Fluency, ToolBox Dimensional Change Card Sort, Flanker Inhibitory Control and Attention Test, Pattern Comparison Processing Speed, List Sorting Working Memory); Memory (RAVLT Total and Delayed Recall, ToolBox Picture Sequence Memory); Visuospatial (WASI Block Design, Judgment of Line Orientation, Facial Recognition); and Motor speed (Grooved Pegboard).
The mean cognitive domain z-scores were then subjected to k-means clustering. Three methods for determining the optimal number of clusters that have shown strong performances relative to other frequently used cluster validity indices in comparison studies (Tibshirani et al., 2001, Vendramin et al., 2010) were examined for cluster sizes up to five. The gap statistic compares the obtained within cluster sum of squared distances from the mean with the expected values under a reference null distribution and the optimal cluster number is the one that maximizes the distance between the observed variance and that obtained with the uniform distribution (Tibshirani et al., 2001). Both the silhouette width statistic (Rousseeuw, 1987) and the PBM index (Pakhira et al., 2004) incorporate both within-group (cohesion) and between-group (separation) distances. The gap statistic and the PBM index suggested a three-cluster solution, while the silhouette width statistic favored a two-cluster solution. Given that two of the three indices suggested an optimal cluster number of three, which is highly interpretable and consistent with prior research in the area, the three-cluster solution was chosen.
Three groups were identified (Fig. 1). Generalized Cognitive Impairment (Generalized-CI) (N = 20, 18% of TLE group) reflecting significant impairment affecting all domains, Focal Cognitive Impairment (Focal-CI) (N = 34, 31%) demonstrated by particularly abnormal language, memory and executive function/processing speed, and No Cognitive Impairment (No-CI) (N = 57, 51%) where performance was intact and comparable to controls across all domains. One-way ANOVAs with post-hoc Games-Howell tests performed in each domain showed that Generalized-CI performed more poorly than all other groups across all domains. Focal-CI performed more poorly than controls in all domains and more poorly than No-CI in all domains except Motor Speed.
Fig. 1.

Cognitive performance of three identified subgroups. Three clusters were identified, with Generalized Cognitive Impairment (Generalized-CI) (red, N = 20) being the most impaired overall, then Focal Cognitive Impairment (Focal-CI) (yellow, N = 34), and No Cognitive Impairment (No-CI) the most intact (blue, N = 57). Error bars represent standard deviations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Sociodemographic and clinical correlates
Cluster groups differed in several sociodemographic and clinical variables (one-way ANOVA for continuous or Fisher Exact Test for categorical variables). P-values were corrected with Benjamini-Hochberg false discovery rate for multiple comparisons. Years of education differed for patients (P < 0.05); patients’ mothers (P = 0.022), with less education in Generalized-CI versus controls (P = 0.041); and fathers’ (P < 0.001), with less education in Generalized-CI (P = 0.002) and Focal-CI (P = 0.017). Age was significantly older in No-CI versus control groups (P = 0.001). Generalized-CI had the largest proportion of non-Caucasian participants (P < 0.001), the presence and greatest number of lifetime generalized tonic-clonic seizures (P < 0.01), and youngest age of first anti-epileptic drug intake (P < 0.05). There were no significant differences between clusters across other demographic (gender, handedness) or clinical seizure variables (age of onset or duration of epilepsy, mono- versus polytherapy).
There was no significant association between cognitive phenotype and laterality defined by interictal EEG (left, right, bilateral) (P = 0.55) or ictal monitoring (P = 0.23). That said, only a subset of TLE participants (34%) underwent ictal monitoring, another indication of the less severe nature of the epilepsy of this cohort. The presence vs absence of ictal monitoring (a potential indication of medication resistant seizures) was not associated with cognitive phenotype membership (P = 0.24).
3.3. Regional analysis
Supplemental File 1 depicts the results for regional analyses of cortical volume and thickness compared to controls. In brief, there were limited focal and not stepwise volume and thickness abnormalities across phenotypes. Generalized-CI and No-CI exhibited reduced cortical thickness primarily in the anterior and inferior temporal lobes bilaterally, while Focal-CI did not differ from controls. Cortical surface volume was increased in Generalized-CI in middle frontal regions bilaterally. Focal-CI demonstrated increased volume restricted to the right middle frontal region. No-CI had increased volume in the left middle frontal region and precentral gyrus, and bilaterally in postcentral gyrus and the precuneus/cuneus.
3.4. Volumetric analyses
Examination of total ICV (ANCOVA with age and gender covariates) was significant (F = 6.4, P < 0.001) with smaller volume compared to controls in Generalized-CI (P = 0.006) and Focal-CI (P = 0.001). Generalized-CI and Focal-CI had smaller left and right cerebellar cortex than No-CI (P = 0.001 and 0.002). There were nonsignificant trends (P > 0.10) of less total gray and white matter in Generalized and Focal CI compared to No-CI and controls. Examination of subcortical volumes (MANCOVA with age, gender, ICV as covariates) revealed significant group effects for left (P < 0.007) and right (P < 0.001) cerebellar cortex. Compared to controls, all TLE groups had smaller left (P’s < 0.05) and right (P’s < 0.02) cerebellar cortex volumes with stepwise declines across phenotype groups.
Adjusted (age, ICV) hippocampal volumes were derived and at a conservative threshold (z ≤ -1.5), 23% of the TLE group exhibited unilateral (12.4%) or bilateral (10.3%) hippocampal atrophy. Using a more liberal threshold (z < -1.0), 43% of the TLE sample exhibited hippocampal atrophy pointing to the presence but less severe nature of hippocampal atrophy in this TLE group. As expected, the rate of hippocampal atrophy (z ≤ -1.5) in the TLE group compared to controls was significantly elevated (P = 0.037). Of those TLE patients with hippocampal atrophy, 55% were unilateral and 45% were bilateral (using z ≤ -1.5 threshold). The association of hippocampal atrophy (none, unilateral, bilateral) with cognitive phenotype distribution was significant (Chi Square = 20.7, df = 9, P < 0.014) indicating that bilateral hippocampal atrophy was associated with the generalized cognitive impairment cluster (No-CI, Focal-CI, Generalized-CI = 10%, 10%, 26%).
3.5. Graph theory analysis
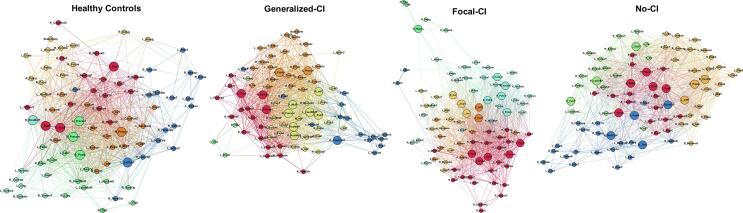
Differences were evident in the morphological networks for each cluster compared to healthy controls (Fig. 2). Even though controls show different modules, they were not as integrated as seen in Focal-CI and Generalized-CI. Interestingly, No-CI presented a community structure more organized than in controls.
Fig. 2.
Community structure of morphological networks. Community structures of healthy controls, Generalized Cognitive Impairment (Generalized-CI), Focal Cognitive Impairment (Focal-CI), and No Cognitive Impairment (No-CI) groups showed differences. Node abbreviations are the same as in Supplementary Table 1. Same color nodes belong to the same module. The spatial distribution of nodes was calculated using the force-atlas graph algorithm, where nodes that demonstrated stronger connections are located closer in space, while nodes with fewer connections tend to be farther apart in space. Bigger nodes represent the hubs of the network using betweenness centrality. Calculated at a hybrid threshold of 25%.
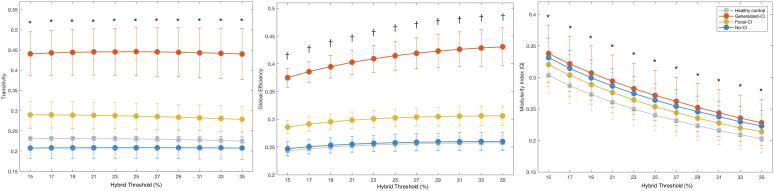
Each TLE cluster showed a significant difference relative to healthy controls for both transitivity (global clustering) and global efficiency, with Generalized-CI the highest, followed by Focal-CI, then controls, and finally No-CI (Fig. 3). Each patient group showed a significant difference relative to healthy controls for both transitivity (global clustering) and modularity index (Q). No-CI was significantly lower than controls regarding transitivity, while Generalized-CI and Focal-CI were both significantly higher than controls. In terms of global efficiency, the only group that was not significantly different from controls was No-CI. Given that No-CI did not differ from controls with regard to cognitive performance, similarities in global efficiency might be beneficial in these patients and a positive trait for cognition. In terms of Q, all three groups were significantly higher than controls, with Generalized-CI the highest, followed by No-CI, and lastly Focal-CI.
Fig. 3.
Comparisons of global measures. Global measures (transitivity, global efficiency, and modularity index) of Generalized Cognitive Impairment (Generalized-CI, red, N = 20), Focal Cognitive Impairment (Focal-CI, yellow, N = 34), and No Cognitive Impairment (No-CI, blue, N = 57) groups showed differences compared to healthy controls (grey). Each group was statistically significant against random at each density level (Bonferroni correction). *Each group statistically significant compared to healthy controls after Bonferroni correction. †The groups of Generalized-CI and Focal-CI statistically significant to healthy controls. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
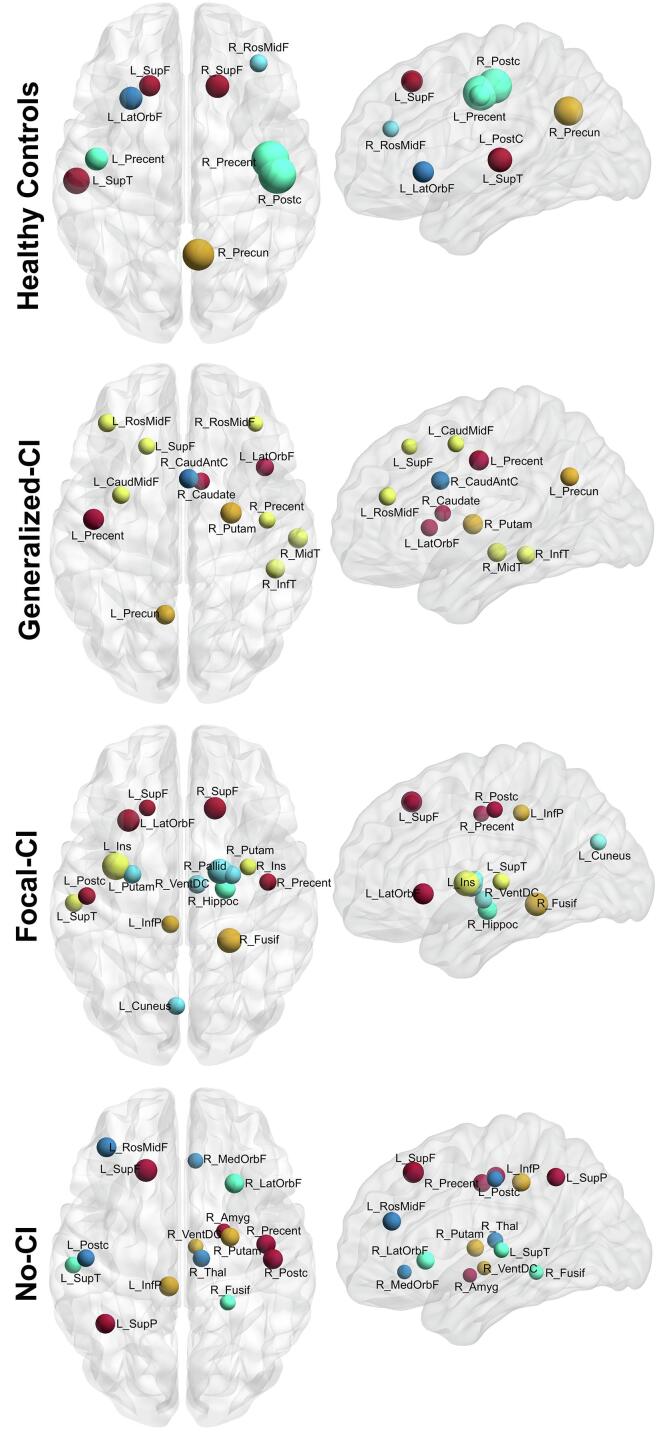
Regarding network hubs (Fig. 4), controls showed six hubs (of nine) from the frontal lobe, two in the parietal lobe (including the right precuneus), and one from temporal areas. All three TLE groups also showed two hubs in the temporal lobe. The group with generalized-CI showed seven hubs (of 13) from the frontal lobe, Focal-CI showed just four (of 16), and No-CI showed five (of 15). Interestingly, all groups of patients showed subcortical hubs. Even though No-CI was the closest to controls in terms of community structure, the distribution of hubs was not as similar: controls having a high proportion in the frontal lobe, whereas No-CI in frontal, parietal, and subcortical areas.
Fig. 4.
Nodes with high betweenness centrality. The pattern of nodes with high betweenness centrality differed among the four groups: (from top to bottom) healthy controls, Generalized Cognitive Impairment (Generalized-CI), Focal Cognitive Impairment (Focal-CI), and No Cognitive Impairment (No-CI) (calculated at a hybrid threshold of 25%). Nodes with the same color represent the same module (as in Fig. 2). Labels are the node abbreviations from Supplementary Table 1.
Given the possible influence of gender and laterality, additional analyses were performed correcting for each of those measures while simultaneously correcting for age and ICV, and they can be found in the Supplemental Files 2 and 3. In summary, adjusting the covariates (from age, to age and gender, to age and laterality) did not substantially alter the results, especially in terms of community structure and the global measures.
3.6. Resting state functional connectivity
The greatest number of significant (corrected P < 0.05) alterations in resting state functional connectivity was found in Generalized-CI, with no significant differences from controls observed for No-CI. Compared to healthy controls, Generalized-CI showed increased correlations (hyperconnectivity) for 167 connections and decreased correlations (hypoconnectivity) for 503 connections (corrected P < 0.05) (Fig. 5). 48 out of 670 were subcortical connections. Left and right Area 10d (anterior cingulate/medial prefrontal cortex); and right Areas 8Av, 8B and 8BL (dorsolateral prefrontal cortex) appeared most frequently (>25 times). Most changes were seen in connections from the bilateral temporal and medial frontal lobes (also see Supplemental File 4 with corrected P < 0.01).
Fig. 5.
Resting-state connectivity changes in temporal lobe epilepsy. Resting-state connectivity changes of temporal lobe epilepsy (TLE) patients in three cognitive impairment (CI) clusters, compared to healthy controls. Red lines indicate decreased connectivity (hypoconnectivity) in the patients, while blue lines indicate increased connectivity (hyperconnectivity). Comparison of controls to the No-CI group did not reveal any significant changes in connectivity after correction. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Focal-CI showed five significant hyperconnectivity and 13 hypoconnectivity differences compared to controls (corrected P < 0.05). Five of the 18 significant differences overlapped with those of Generalized-CI. No-CI showed no significant differences from controls after correction.
Regarding potential impact of laterality on the findings, the resting state analyses compared cluster groups to controls, so laterality could not be entered as a covariate of no interest as it does not apply to controls. We assessed whether there were significant differences in resting state connectivity between TLE laterality groups. This was done first using subjects classified as left, right and bilateral, then with just left and right lateralization. There were no significant findings following correction (P > 0.15).
4. Discussion
This investigation reveals a taxonomy of cognitive abnormality in TLE that only partially overlaps with expectations based on syndrome-specific pathophysiology, the cognitive phenotypes influenced by diverse epilepsy, sociodemographic, and neuroimaging features reflecting the combined influence of socioeconomic, neurodevelopmental and neurobiological factors. First, while the overall cognitive profile of participants with TLE revealed generalized cognitive impairment consistent with prior reports, three specific subgroups were identified that differed significantly in their presenting cognitive profiles, consistent with an underlying cognitive taxonomy with two of the cognitive clusters substantially independent of the primary underlying pathophysiology. Second, several interesting and not previously reported clinical and sociodemographic factors were associated with these cognitive subtypes. Third, traditional neuroimaging markers of structural abnormality (e.g., cortical volume) were less related to the cognitive phenotypes than indices of connectivity (resting state) and broad network analyses of cortical and subcortical volumes. Each of these points will be addressed below.
4.1. Cognitive profiles
The average cognitive profile of the TLE participants suggested generalized impairment, consistent with prior reports (Guimaraes et al., 2007, Hwang et al., 2019, Marques et al., 2007, Oyegbile et al., 2004, Rzezak et al., 2007), reflected in their abnormal performance compared to controls across all five cognitive domains. In that context, the hypothesis regarding underlying cognitive phenotypes was again confirmed, as the analytic approach identified three subgroups characterized by divergent neuropsychological profiles. Notable, and generally underappreciated, was the large subset of epilepsy participants whose cognitive status was indistinguishable from healthy controls across all domains (No-CI), representing 51% of the cohort. The presence and proportion of this group is important as it provides a contrast to the view of TLE as a generally cognitively impairing disorder, instead suggesting that a spectrum of cognitive presentations exist including a completely intact group. To that point, the other notable group was the significant minority of patients with a heavy cognitive burden who demonstrated significantly impaired performance across all cognitive domains (Generalized-CI), representing 18% of this “focal” epilepsy cohort. In spite of a focal epilepsy syndrome, cognition was globally and severely impacted, affecting memory and all other tested cognitive domains. The remainder (Focal-CI), representing 31% of the sample, exhibited mildly depressed performance across several cognitive domains but were especially impacted in expected domains including language, memory, and executive function. Thus, there is a systematic taxonomy of cognition embedded within this common focal epilepsy.
Important directions for future research include determining whether other epilepsy syndromes (e.g., frontal lobe epilepsy, juvenile myoclonic epilepsy) also harbor discrete cognitive phenotypes and whether there are shared phenotypes across diverse epilepsy syndromes (e.g., intact, generalized impairment, syndrome-specific phenotype). That this may be the case can be inferred by neuroimaging findings from ENIGMA-Epilepsy (Whelan et al., 2018), a large-scale, global initiative including 2149 epilepsy patients and 1727 healthy controls. Identified were substantial regions of shared cortical and subcortical atrophy as well as shared microstructural alterations across groups of patients with TLE, extratemporal epilepsy and genetic generalized epilepsy, in addition to syndrome-specific structural abnormalities.
Comparing the current findings to our original contribution (Hermann et al., 2007) there are several similarities. Both investigations identified three cognitive clusters including “intact” and “generalized impaired” clusters, as well as a third cluster with “focal” cognitive impairments. In the 2007 paper the “focal” group was prominently impaired in immediate and delayed memory, but that group was also significantly lower (albeit mildly) than controls across all other cognitive domains including intelligence, language, perception, executive function and speed. Similarly, here the focal group shows abnormalities in memory as well as language and executive function. One might question whether “focal” is the best term for this group as several domains are impacted and better might be “expected for TLE”.
4.2. Cognitive phenotype associations
4.2.1. Sociodemographic and clinical factors
Years of education is considered a marker of “cognitive reserve”, with less education linked to increased risk for age-related cognitive change/decline and cognitive risk in the face of neurological and medical insults (Stern, 2009, Stern et al., 2018). Here the results were unexpected. Education was significantly different across groups, not only for TLE patients but also for their mothers and fathers as well, with education lowest in Generalized-CI. “Limited reserve” (i.e., less education) appears not to be a characteristic consequent to epilepsy, but a shared familial characteristic which may reflect socioeconomic impact and risk (Glosser et al., 1997). In general, lower education has been reported to be associated with the more impaired cognitive phenotype in other investigations (Elverman et al., 2019, Rodríguez-Cruces et al., 2018) suggesting that parsing the presence and broader correlates of low personal and familial education is in order. In addition, racial diversity separated the cognitive phenotypes with the greatest diversity in Generalized-CI, another potential socioeconomic indicator.
Among clinical epilepsy factors both the presence and the number of lifetime generalized seizures and age of first anti-epileptic drug intake discriminated the groups with more lifetime seizures and earliest age of anti-epileptic drug treatment in the most impaired cluster (Generalized-CI). Lifetime generalized tonic-clonic seizures has long been of interest in the neuropsychology of epilepsy, linked to early reports of increased risk of clinician-rated mental handicap (Lennox, 1960) and later related to objective neuropsychological test performance (Dodrill and Matthews, 1992). While these empirical associations are informative, causality remains to be determined—with particular interest in the contribution of socioeconomic disadvantage which may impact the patient and family more broadly.
There were no significant differences between clusters across other demographic (gender, handedness) or clinical seizure variables (age of onset or duration of epilepsy, laterality of EEG focus, need for ictal monitoring, mono- versus polytherapy).
4.2.2. Neuroimaging correlates of phenotypes
The underlying neurobiology of the cognitive phenotypes was explored using traditional regional (vertex-based) examination of cortical volume and thickness as well as volumes of subcortical and cerebellar volumes, network analysis of large-scale patterns of subcortical and cortical covariance in gray matter volume—a more dynamic and systems-based approach with which to interrogate relationships with cognition, and rs-fMRI directly assessing disruptions in connectivity.
4.2.2.1. Structural correlates
The cognitive phenotypes revealed only a modest association with traditional regional metrics of cortical volume and thickness, and specifically not observed was a “stair-step” association between the worsening cognitive impairment and vertex results. In our earlier paper using a largely treatment resistant group and a Freesurfer analysis of the clusters (Dabbs et al., 2009), an orderly relationship existed between cluster group and the degree of abnormality in cortical thickness. That relationship was not observed here which in part we suspect may be related to the nature of the ECP cohort as described previously (Section 2.1, Participants). In that TLE is a network disorder and the ECP sample represents a more benign profile than our previous refractory group, regional thickness/volume estimates may not be sensitive enough, whereas the GT measures are able to capture group differences. In point of fact, recent work with a specific “language-impaired phenotype” showed that regional measures of superficial white matter compromise did not reveal differences between the language-impaired phenotype and healthy controls, but network-based measures did (Kaestner et al., 2019).
Most evident were differences between controls and cluster groups in estimated ICV (adjusted for age and gender), a reflection of ultimate brain development and viewed as a marker of brain reserve (van Loenhoud et al., 2018). Total ICV differed between controls and Generalized-CI and Focal-CI groups, but not No-CI. Imaging based markers of brain reserve are uncommonly examined in the epilepsy literature.
There was also an association between the presence of bilateral hippocampal atrophy and cluster membership with an increased rate of atrophy in the Generalized-CI group. Previous analyses examining the link between hippocampal pathology to cognitive phenotype have been mixed with some reporting positive findings (Rodríguez-Cruces et al., 2018) and others negative results (Elverman et al., 2019, Reyes et al., 2019). The studies have varied in underlying methods and definitions which may have contributed to this variability.
There were also differences between groups in left and right cerebellar cortex volumes with controls exhibiting greater volume compared to all cluster groups. Cerebellar atrophy is a known complication of the chronic epilepsies including TLE (Hagemann et al., 2002, Hermann et al., 2005, Oyegbile et al., 2011); linked to iatrogenic medication effects and the severity of epilepsy (e.g., lifetime generalized seizures) (Hagemann et al., 2002), and associated with cognitive morbidity (Botez et al., 1989, Dabbs et al., 2009). The cerebellum has a protracted development during which it is vulnerable to multiple biological and environmental insults (Tiemeier et al., 2010) and it is possible for atrophy to be out of proportion to the degree of cortical atrophy (Hermann et al., 2005).
Relatedly, there is a substantial literature demonstrating associations between socioeconomic status (SES) and brain structure and development involving multiple brain regions, including cerebellum (Cavanagh et al., 2013, Conant et al., 2017, Farah, 2017, Farah, 2018, Jenkins et al., 2019, Kim et al., 2019, Leijser et al., 2018). To our knowledge, total ICV has not been examined in relation to economic or social disadvantage.
4.2.2.2. Graph theory analysis
While vertex-based analyses of cortical volume and thickness did not reveal progressive abnormalities as a function of worsening cognition (i.e., cluster membership), when brain volumes were analyzed via network science techniques, interesting and regular patterns associated with the cognitive phenotypes were detected. Specifically, No-CI showed a configuration of morphological covariance networks similar to the healthy controls. Modules in Focal-CI and No-CI seemed more integrated than in controls or Generalized-CI, which suggested a benefit from increased correlations of cortical and subcortical volumes. In addition, Focal-CI and Generalized-CI showed higher global clustering and efficiency compared to controls, while No-CI was lower and clearly distinct from the others. Although No-CI presented a global configuration similar to controls, it still did not resemble controls in regard to node-specific measures. These regional dissimilarities indicate an underlying morphological development that might have been beneficial for the cognitive superiority of No-CI with respect to the other cluster groups.
Although regional and network-based analyses were performed using the same morphological data (i.e., volumetric data), the nature of graph theory analyses provided another view of morphometric data that rendered additional insights regarding how the cognitive phenotypes differed in their morphological associations. This same pattern was observed in a cohort of patients with uncomplicated childhood onset epilepsies 50 years after their diagnosis (Garcia-Ramos et al., 2017), where graph theory analyses of the morphological networks revealed subtleties missed by conventional regional analyses. Further, Reyes et al. (2019) recently demonstrated that TLE patients with an isolated language impairment showed unremarkable regional analyses but differences in network structure using graph theory analysis (Reyes et al., 2019).
4.2.2.3. Resting state connectivity
Similarly, the analyses of resting state connectivity revealed an orderly set of findings across the cognitive phenotype groups. No-CI showed no significant connectivity differences compared to the controls. Generalized-CI showed the most extensive connectivity differences (670 out of 71,631 connections after FDR correction), while abnormality in Focal-CI was present but less extensive (18 connections) (Fig. 5 and Supplementary Fig. 1). While the connectivity abnormalities lack pattern in Focal-CI, a large proportion of abnormalities in Generalized-CI are from the medial frontal and temporal lobe areas implicating notable dysconnectivity between these regions.
4.3. Conclusions
Patients with TLE exhibit a cognitive taxonomy consistent with the presence of clinically important cognitive subtypes of TLE with neurobiological, clinical and sociodemographic correlates. Only one of the three cognitive phenotypes bore a resemblance to the classic disordered pathophysiology of TLE (Focal-CI). Differences in total ICV, smallest in the most impaired cluster (Generalized-CI), raises the question of influence of an adverse neurodevelopmental impact as does the earlier age at medication treatment in this cluster. Variations in patient and parental education (lowest in Generalized-CI) and racial/ethnic diversity (highest in Generalized-CI) raise the possibility of adverse influences linked to lower SES. The possibility that early disadvantage contributed to smaller ICV in Generalized-CI cannot be ruled out. Population-based research has demonstrated higher rates of epilepsy in disadvantaged populations, the disadvantage not due to social drift (Magnusson and Zelano, 2019, Pickrell et al., 2015, Steer et al., 2014), and racial and SES factors have been shown to influence features of epilepsy presentation (Allen et al., 2018, Allen et al., 2019). As disadvantage is known to impact cognition, brain and behavior in the general population, it appears important to more seriously consider these factors in the neuropsychology of epilepsy (Baxendale and Heaney, 2011).
Despite the largely unrevealing regional analyses of cortical gray volume and thickness, subsequent analyses pointed to disordered “networks” within cortical and subcortical gray matter with altered patterns of connectivity demonstrated by graph theory and rs-fMRI analyses, along with significant abnormalities in cerebellar cortex volume. Systematic variations in morphological covariance networks and node specific metrics (graph theory analyses) in conjunction with alterations in temporal-frontal connectivity across the groups speaks to the importance of disordered systems neurobiology underlying the cognitive taxonomy of TLE, particularly in this arguably representative (non-surgical) sample of TLE patients, althoughs the cognitive patterns replicate those observed in group of mostly refractory patients (Hermann et al., 2007). However, network-based analyses may be even more important for understanding cognitive impairment in these less intractable cases as their impairments are “unexplained” by traditional regional atrophy metrics.
Lastly, Generalized-CI would appear to be at additional cognitive risk in the face of increasing chronological age given their reduced brain (ICV) and cognitive reserve, midlife cognitive compromise, and contributions of known epilepsy-related risk factors (lifetime generalized seizures).
These results build upon our prior findings not only by replicating the presence and nature of underlying cognitive phenotypes in a less severely affected group of patients with TLE, but by expanding the search for potential correlates to new areas of neuroimaging (rs-fMRI) and the nature and sophistication of imaging analyses (network science), with inclusion of sociodemographic factors that have been significantly understudied as predictors in the epilepsy-cognition literature, and the use of contemporary cognitive metrics from the NIH ToolBox Cognitive Battery.
4.4. Limitations and future directions
This investigation has limitations. The investigation is cross-sectional which places limits on the ability to advance causal explanations for reported findings, but testable hypotheses for future longitudinal studies are clear. As noted, a weakness here is the lack of ictal monitoring for all TLE patients, but a strength is the broader representativeness of this sample of TLE patients beyond the medically intractable. Overall, across TLE patients of varying severity this emerging literature has pointed consistently to a definable cognitive taxonomy (Elverman et al., 2019, Reyes et al., 2019, Rodríguez-Cruces et al., 2018). As studies of this type expand a fuller vision of the determinants will evolve and examination of other epilepsy syndromes will further inform the cognitive taxonomy of the epilepsies.
Funding
This study was supported by grant number U01NS093650 (Epilepsy Connectome Project) from the National Institutes of Health. Supplemental funding for healthy control subjects’ data acquisition was provided in part by the Departments of Radiology and Neurology at the University of Wisconsin School of Medicine and Public Health. Charlene N. Rivera-Bonet was supported in part by grant number T32MH018931-30 from the National Institutes of Mental Health. Cole J Cook was supported in part by grant number T32CA009206 from the National Cancer Institute of the National Institutes of Health.
CRediT authorship contribution statement
Bruce Hermann: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing - original draft, Writing - review & editing. Lisa L. Conant: Conceptualization, Funding acquisition, Formal analysis, Investigation, Methodology, Validation, Writing - original draft, Writing - review & editing. Cole J. Cook: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing - original draft, Writing - review & editing. Gyujoon Hwang: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Camille Garcia-Ramos: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Kevin Dabbs: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Veena A. Nair: Data curation, Investigation, Project administration, Supervision, Validation, Writing - review & editing. Jedidiah Mathis: Investigation, Validation, Writing - review & editing. Charlene N. Rivera Bonet: Investigation, Validation, Writing - review & editing. Linda Allen: Investigation, Validation, Writing - review & editing. Dace N. Almane: Investigation, Validation, Writing - review & editing. Karina Arkush: Resources, Writing - review & editing. Rasmus Birn: Investigation, Validation, Writing - review & editing. Edgar A. DeYoe: Investigation, Validation, Writing - review & editing. Elizabeth Felton: Investigation, Validation, Writing - review & editing. Rama Maganti: Investigation, Validation, Writing - review & editing. Andrew Nencka: Investigation, Validation, Writing - review & editing. Manoj Raghavan: Investigation, Validation, Writing - review & editing. Umang Shah: Resources, Writing - review & editing. Veronica N. Sosa: Resources, Writing - review & editing. Aaron F. Struck: Investigation, Validation, Writing - review & editing. Candida Ustine: Investigation, Validation, Writing - review & editing. Anny Reyes: Validation, Writing - original draft. Erik Kaestner: Validation, Writing - original draft. Carrie McDonald: Validation, Writing - original draft. Vivek Prabhakaran: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing - review & editing. Jeffrey R. Binder: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing - review & editing. Mary E. Meyerand: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank all the participants and their families. Additionally, the authors would like to thank Megan Rozman, Taylor McMillan, Elizabeth Awe, Courtney Forseth, Peter Kraegel, Anna Freiberg, Neelima Tellapragada for recruitment and data collection, MRI technologists for their assistance in scanning and other support staff.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102341.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.

Resting state connectivity changes in Generalized Cognitive Impairment group. This figure shows significant resting state connectivity changes of Generalized Cognitive Impairment (Generalized-CI) groups, compared to healthy controls at a higher adjusted threshold (corrected P < 0.01). Red lines indicate decreased connectivity (hypoconnectivity) in the patients, while blue lines indicate increased connectivity (hyperconnectivity).
Regional analyses of cortical volume and thickness.
Correcting for age and sex.
Correcting for age and laterality.
Nodes used in graph theory.
References
- Allen S.E., Limdi N., Westrick A.C., Ver Hoef L.W., Szaflarski J.P., Knowlton R.C. Racial disparities in temporal lobe epilepsy. Epilepsy Res. 2018;140:56–60. doi: 10.1016/j.eplepsyres.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Allen S.E., Limdi N.A., Westrick A.C., Ver Hoef L.W., Szaflarski J.P., Kuzniecky R.I., Knowlton R.C. Racial differences in adult-onset MRI-negative temporal lobe epilepsy. Epilepsy Behav. 2019;100(Pt A) doi: 10.1016/j.yebeh.2019.106501. [DOI] [PubMed] [Google Scholar]
- Baxendale S., Heaney D. Socioeconomic status, cognition, and hippocampal sclerosis. Epilepsy Behav. 2011;20(1):64–67. doi: 10.1016/j.yebeh.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Baxendale S., Thompson P. Beyond localization: the role of traditional neuropsychological tests in an age of imaging. Epilepsia. 2010;51(11):2225–2230. doi: 10.1111/j.1528-1167.2010.02710.x. [DOI] [PubMed] [Google Scholar]
- Benton A.L., Hamsher K.D., Varney N.R., Spreen O. Oxford University Press; New York, NY: 1983. Contributions to Neuropsychological Assessment: A Clinical Manual. [Google Scholar]
- Blondel V.D., Guillaume J.L., Lambiotte R., Lefebvre E. Fast unfolding of communities in large networks. J. Statistical Mech.-Theory Exp. 2008 doi: 10.1088/1742-5468/2008/10/P10008. [DOI] [Google Scholar]
- Boccaletti S., Latora V., Moreno Y., Chavez M., Hwang D.U. Complex networks: structure and dynamics. Phys. Rep.-Rev. Section Phys. Lett. 2006;424(4–5):175–308. doi: 10.1016/j.physrep.2005.10.009. [DOI] [Google Scholar]
- Botez M.I., Botez T., Elie R., Attig E. Role of the cerebellum in complex human behavior. Ital. J. Neurol. Sci. 1989;10(3):291–300. doi: 10.1007/bf02333774. [DOI] [PubMed] [Google Scholar]
- Braakman H.M., Vaessen M.J., Jansen J.F., Debeij-van Hall M.H., de Louw A., Hofman P.A., Backes W.H. Aetiology of cognitive impairment in children with frontal lobe epilepsy. Acta Neurol. Scand. 2015;131(1):17–29. doi: 10.1111/ane.12283. [DOI] [PubMed] [Google Scholar]
- Braeken J., van Assen M. An empirical Kaiser criterion. Psychol. Methods. 2017;22(3):450–466. doi: 10.1037/met0000074. [DOI] [PubMed] [Google Scholar]
- Bremm F.J., Hendriks M.P.H., Bien C.G., Grewe P. Pre- and postoperative verbal memory and executive functioning in frontal versus temporal lobe epilepsy. Epilepsy Behav. 2019;101(Pt A) doi: 10.1016/j.yebeh.2019.106538. [DOI] [PubMed] [Google Scholar]
- Carlozzi N.E., Beaumont J.L., Tulsky D.S., Gershon R.C. The NIH toolbox pattern comparison processing speed test: normative data. Arch. Clin. Neuropsychol. 2015;30(5):359–368. doi: 10.1093/arclin/acv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi N.E., Tulsky D.S., Chiaravalloti N.D., Beaumont J.L., Weintraub S., Conway K., Gershon R.C. NIH Toolbox Cognitive Battery (NIHTB-CB): the NIHTB Pattern Comparison Processing Speed Test. J Int Neuropsychol Soc. 2014;20(6):630–641. doi: 10.1017/S1355617714000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J., Krishnadas R., Batty G.D., Burns H., Deans K.A., Ford I., McLean J. Socioeconomic status and the cerebellar grey matter volume. Data from a well-characterised population sample. Cerebellum. 2013;12(6):882–891. doi: 10.1007/s12311-013-0497-4. [DOI] [PubMed] [Google Scholar]
- Conant L.L., Liebenthal E., Desai A., Binder J.R. The relationship between maternal education and the neural substrates of phoneme perception in children: Interactions between socioeconomic status and proficiency level. Brain Lang. 2017;171:14–22. doi: 10.1016/j.bandl.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant L.L., Wilfong A., Inglese C., Schwarte A. Dysfunction of executive and related processes in childhood absence epilepsy. Epilepsy Behav. 2010;18(4):414–423. doi: 10.1016/j.yebeh.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Cook C.J., Hwang G., Mathis J., Nair V.A., Conant L.L., Allen L., Meyerand M.E. Effective connectivity within the default mode network in left temporal lobe epilepsy: findings from the epilepsy connectome project. Brain Connect. 2019;9(2):174–183. doi: 10.1089/brain.2018.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crawford A.V., Green S.B., Levy R., Lo W.J., Scott L., Svetina D., Thompson M.S. Evaluation of parallel analysis methods for determining the number of factors. Educ. Psychol. Measur. 2010;70(6):885–901. doi: 10.1177/0013164410379332. [DOI] [Google Scholar]
- Dabbs K., Jones J., Seidenberg M., Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav. 2009;15(4):445–451. doi: 10.1016/j.yebeh.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dodrill C.B., Matthews C.G. The role of neuropsychology in the assessment and treatment of persons with epilepsy. Am. Psychol. 1992;47(9):1139–1142. doi: 10.1037//0003-066x.47.9.1139. [DOI] [PubMed] [Google Scholar]
- Elger C.E., Helmstaedter C., Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3(11):663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Elverman K.H., Resch Z.J., Quasney E.E., Sabsevitz D.S., Binder J.R., Swanson S.J. Temporal lobe epilepsy is associated with distinct cognitive phenotypes. Epilepsy Behav. 2019;96:61–68. doi: 10.1016/j.yebeh.2019.04.015. [DOI] [PubMed] [Google Scholar]
- Farah M.J. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron. 2017;96(1):56–71. doi: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Farah M.J. Socioeconomic status and the brain: prospects for neuroscience-informed policy. Nat. Rev. Neurosci. 2018;19(7):428–438. doi: 10.1038/s41583-018-0023-2. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fonseca Wald E.L.A., Hendriksen J.G.M., Drenthen G.S., Kujik S.M.J.V., Aldenkamp A.P., Vles J.S.H., Klinkenberg S. Towards a better understanding of cognitive deficits in absence epilepsy: a systematic review and meta-analysis. Neuropsychol. Rev. 2019;29(4):421–449. doi: 10.1007/s11065-019-09419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramos C., Bobholz S., Dabbs K., Hermann B., Joutsa J., Rinne J.O., Group T.S. Brain structure and organization five decades after childhood onset epilepsy. Hum. Brain Mapp. 2017;38(6):3289–3299. doi: 10.1002/hbm.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramos C., Lin J.J., Bonilha L., Jones J.E., Jackson D.C., Prabhakaran V., Hermann B.P. Disruptions in cortico-subcortical covariance networks associated with anxiety in new-onset childhood epilepsy. Neuroimage-Clinical. 2016;12:815–824. doi: 10.1016/j.nicl.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramos C., Lin J.J., Prabhakaran V., Hermann B.P. Developmental reorganization of the cognitive network in pediatric epilepsy. PLoS ONE. 2015;10(10) doi: 10.1371/journal.pone.0141186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Coalson T.S., Robinson E.C., Hacker C.D., Harwell J., Yacoub E., Van Essen D.C. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Consortium W.U.-M.H. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorfeld L.W. An improvement on horns parallel analysis methodology for selecting the correct number of factors to retain. Educ. Psychol. Measur. 1995;55(3):377–393. doi: 10.1177/0013164495055003002. [DOI] [Google Scholar]
- Glosser G., Cole L.C., French J.A., Saykin A.J., Sperling M.R. Predictors of intellectual performance in adults with intractable temporal lobe epilepsy. J. Int. Neuropsychol. Soc. 1997;3(3):252–259. [PubMed] [Google Scholar]
- Guimaraes C.A., Li L.M., Rzezak P., Fuentes D., Franzon R.C., Augusta Montenegro M., Guerreiro M.M. Temporal lobe epilepsy in childhood: comprehensive neuropsychological assessment. J. Child Neurol. 2007;22(7):836–840. doi: 10.1177/0883073807304701. [DOI] [PubMed] [Google Scholar]
- Hagemann G., Lemieux L., Free S.L., Krakow K., Everitt A.D., Kendall B.E., Shorvon S.D. Cerebellar volumes in newly diagnosed and chronic epilepsy. J. Neurol. 2002;249(12):1651–1658. doi: 10.1007/s00415-002-0843-9. [DOI] [PubMed] [Google Scholar]
- Heaton R.K., Miller S.W., Taylor M.J., Grant I. Psychological Assessment Resources Inc; Lutz: 2004. Revised comprehensive norms for an expanded halstead reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. [Google Scholar]
- Helmstaedter C., Witt J.A. Multifactorial etiology of interictal behavior in frontal and temporal lobe epilepsy. Epilepsia. 2012;53:1765–1773. doi: 10.1111/j.1528-1167.2012.03602.x. [DOI] [PubMed] [Google Scholar]
- Hermann B.P., Bayless K., Hansen R., Parrish J., Seidenberg M. Cerebellar atrophy in temporal lobe epilepsy. Epilepsy Behav. 2005;7(2):279–287. doi: 10.1016/j.yebeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Hermann B.P., Seidenberg M., Lee E.J., Chan F., Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J. Int. Neuropsychol. Soc. 2007;13(1):12–20. doi: 10.1017/S135561770707004X. [DOI] [PubMed] [Google Scholar]
- Hodge M.R., Horton W., Brown T., Herrick R., Olsen T., Hileman M.E., Marcus D.S. ConnectomeDB–Sharing human brain connectivity data. Neuroimage. 2016;124(Pt B):1102–1107. doi: 10.1016/j.neuroimage.2015.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoinkiss D.C., Erhard P., Breutigam N.J., van Samson-Himmelstjerna F., Gunther M., Porter D.A. Prospective motion correction in functional MRI using simultaneous multislice imaging and multislice-to-volume image registration. Neuroimage. 2019;200:159–173. doi: 10.1016/j.neuroimage.2019.06.042. [DOI] [PubMed] [Google Scholar]
- Hwang G., Dabbs K., Conant L., Nair V.A., Mathis J., Almane D.N., Hermann B. Cognitive slowing and its underlying neurobiology in temporal lobe epilepsy. Cortex. 2019;117:41–52. doi: 10.1016/j.cortex.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D.C., Dabbs K., Walker N.M., Jones J.E., Hsu D.A., Stafstrom C.E., Hermann B.P. The neuropsychological and academic substrate of new/recent-onset epilepsies. J. Pediatr. 2013;162(5):1047–1053. doi: 10.1016/j.jpeds.2012.10.046. e1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L.M., Chiang J.J., Vause K., Hoffer L., Alpert K., Parrish T.B., Miller G.E. Subcortical structural variations associated with low socioeconomic status in adolescents. Hum. Brain Mapp. 2019 doi: 10.1002/hbm.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kaestner E., Reyes A., Macari A.C., Chang Y.H., Paul B.M., Hermann B.P., McDonald C.R. Identifying the neural basis of a language-impaired phenotype of temporal lobe epilepsy. Epilepsia. 2019;60(8):1627–1638. doi: 10.1111/epi.16283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E.F., Goodglass H., Weintraub S. 2nd ed. Lea & Febiger; Philadelphia, PA: 1983. The Boston Naming Test. [Google Scholar]
- Keller S.S., Richardson M.P., O'Muircheartaigh J., Schoene-Bake J.C., Elger C., Weber B. Morphometric MRI alterations and postoperative seizure control in refractory temporal lobe epilepsy. Hum. Brain Mapp. 2015;36(5):1637–1647. doi: 10.1002/hbm.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.J., Davis E.P., Sandman C.A., Glynn L., Sporns O., O'Donnell B.F., Hetrick W.P. Childhood poverty and the organization of structural brain connectome. Neuroimage. 2019;184:409–416. doi: 10.1016/j.neuroimage.2018.09.041. [DOI] [PubMed] [Google Scholar]
- Klove H. Clinical neuropsychology. Med. Clin. North Am. 1963;47:1647–1658. [PubMed] [Google Scholar]
- Kwan P., Brodie M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Leijser L.M., Siddiqi A., Miller S.P. Imaging evidence of the effect of socio-economic status on brain structure and development. Semin. Pediatr. Neurol. 2018;27:26–34. doi: 10.1016/j.spen.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Lencz T., McCarthy G., Bronen R.A., Scott T.M., Inserni J.A., Sass K.J., Spencer D.D. Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann. Neurol. 1992;31(6):629–637. doi: 10.1002/ana.410310610. [DOI] [PubMed] [Google Scholar]
- Lennox, W.G., 1960. Epilepsy and related disorders: Little, Brown.
- Lin J.J., Mula M., Hermann B.P. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012;380(9848):1180–1192. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.J., Salamon N., Lee A.D., Dutton R.A., Geaga J.A., Hayashi K.M., Thompson P.M. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb. Cortex. 2007;17(9):2007–2018. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- Loring D.W. History of neuropsychology through epilepsy eyes. Arch. Clin. Neuropsychol. 2010;25(4):259–273. doi: 10.1093/arclin/acq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughman A., Bowden S.C., D'Souza W. Cognitive functioning in idiopathic generalised epilepsies: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2014;43:20–34. doi: 10.1016/j.neubiorev.2014.02.012. [DOI] [PubMed] [Google Scholar]
- MacAllister W.S., Schaffer S.G. Neuropsychological deficits in childhood epilepsy syndromes. Neuropsychol. Rev. 2007;17(4):427–444. doi: 10.1007/s11065-007-9048-4. [DOI] [PubMed] [Google Scholar]
- Magnusson C., Zelano J. High-resolution mapping of epilepsy prevalence, ambulance use, and socioeconomic deprivation in an urban area of Sweden. Epilepsia. 2019;60(10):2060–2067. doi: 10.1111/epi.16339. [DOI] [PubMed] [Google Scholar]
- Marques C.M., Caboclo L.O., da Silva T.I., Noffs M.H., Carrete H., Jr., Lin K., Yacubian E.M. Cognitive decline in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsy Behav. 2007;10(3):477–485. doi: 10.1016/j.yebeh.2007.02.002. [DOI] [PubMed] [Google Scholar]
- McDonald C.R., Hagler D.J., Jr., Ahmadi M.E., Tecoma E., Iragui V., Gharapetian L., Halgren E. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia. 2008;49(5):794–803. doi: 10.1111/j.1528-1167.2008.01539.x. [DOI] [PubMed] [Google Scholar]
- Moeller S., Yacoub E., Olman C.A., Auerbach E., Strupp J., Harel N., Ugurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010;63(5):1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri M.L., Guimaraes C.A., Oliveira E.P., Duran M.H., Medeiros L.L., Montenegro M.A., Guerreiro M.M. Neuropsychological assessment of children with rolandic epilepsy: executive functions. Epilepsy Behav. 2012;24(4):403–407. doi: 10.1016/j.yebeh.2012.04.131. [DOI] [PubMed] [Google Scholar]
- Nickels K.C., Zaccariello M.J., Hamiwka L.D., Wirrell E.C. Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat. Rev. Neurol. 2016;12(8):465–476. doi: 10.1038/nrneurol.2016.98. [DOI] [PubMed] [Google Scholar]
- Novelly R.A. The debt of neuropsychology to the epilepsies. Am. Psychol. 1992;47(9):1126–1129. doi: 10.1037//0003-066x.47.9.1126. [DOI] [PubMed] [Google Scholar]
- Nuyts S., D'Souza W., Bowden S.C., Vogrin S.J. Structural brain abnormalities in genetic generalized epilepsies: a systematic review and meta-analysis. Epilepsia. 2017;58(12):2025–2037. doi: 10.1111/epi.13928. [DOI] [PubMed] [Google Scholar]
- Otte W.M., van Eijsden P., Sander J.W., Duncan J.S., Dijkhuizen R.M., Braun K.P. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia. 2012;53(4):659–667. doi: 10.1111/j.1528-1167.2012.03426.x. [DOI] [PubMed] [Google Scholar]
- Oyegbile T.O., Bayless K., Dabbs K., Jones J., Rutecki P., Pierson R., Hermann B. The nature and extent of cerebellar atrophy in chronic temporal lobe epilepsy. Epilepsia. 2011;52(4):698–706. doi: 10.1111/j.1528-1167.2010.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyegbile T.O., Dow C., Jones J., Bell B., Rutecki P., Sheth R., Hermann B.P. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62(10):1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- Pakhira M.K., Bandyopadhyay S., Maulik U. Validity index for crisp and fuzzy clusters. Pattern Recogn. 2004;37(3):487–501. doi: 10.1016/j.patcog.2003.06.005. [DOI] [Google Scholar]
- Patriat R., Molloy E.K., Meier T.B., Kirk G.R., Nair V.A., Meyerand M.E., Birn R.M. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. Neuroimage. 2013;78:463–473. doi: 10.1016/j.neuroimage.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell W.O., Lacey A.S., Bodger O.G., Demmler J.C., Thomas R.H., Lyons R.A., Kerr M.P. Epilepsy and deprivation, a data linkage study. Epilepsia. 2015;56(4):585–591. doi: 10.1111/epi.12942. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch R. Anatomical substrates of interictal memory deficits in temporal lobe epileptics. Int. J. Neurol. 1987;21–22:17–32. [PubMed] [Google Scholar]
- Rayner G., Tailby C. Current concepts of memory disorder in epilepsy: edging towards a network account. Curr. Neurol. Neurosci. Rep. 2017;17(8):55. doi: 10.1007/s11910-017-0765-7. [DOI] [PubMed] [Google Scholar]
- Rayner G., Tailby C., Jackson G., Wilson S. Looking beyond lesions for causes of neuropsychological impairment in epilepsy. Neurology. 2019;92(7):e680–e689. doi: 10.1212/WNL.0000000000006905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey, A., 1964. L'Examen clinique en psychologie , par André Rey,... 2e édition. Paris: Presses universitaires de France (Vendôme Impr. des P.U.F.).
- Reyes A., Kaestner E., Bahrami N., Balachandra A., Hegde M., Paul B.M., McDonald C.R. Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities. Neurology. 2019;92(17):e1957–e1968. doi: 10.1212/WNL.0000000000007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Cruces R., Bernhardt B.C., Concha L. Multidimensional associations between cognition and connectome organization in temporal lobe epilepsy. Neuroimage. 2020 Jun;213 doi: 10.1016/j.neuroimage.2020.116706. Epub 2020 Mar 6. PMID: 32151761. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Cruces R., Velázquez-Pérez L., Rodríguez-Leyva I., Velasco A.L., Trejo-Martínez D., Barragán-Campos H.M., Concha L. Association of white matter diffusion characteristics and cognitive deficits in temporal lobe epilepsy. Epilepsy Behav. 2018;79:138–145. doi: 10.1016/j.yebeh.2017.11.040. [DOI] [PubMed] [Google Scholar]
- Rousseeuw P.J. Silhouettes – a graphical aid to the interpretation and validation of cluster-analysis. J. Comput. Appl. Math. 1987;20:53–65. doi: 10.1016/0377-0427(87)90125-7. [DOI] [Google Scholar]
- Rudzinski L.A., Meador K.J. Epilepsy and neuropsychological comorbidities. Continuum (Minneap Minn) 2013;19(3):682–696. doi: 10.1212/01.CON.0000431382.06438.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzezak P., Fuentes D., Guimaraes C.A., Thome-Souza S., Kuczynski E., Li L.M., Valente K.D. Frontal lobe dysfunction in children with temporal lobe epilepsy. Pediatr. Neurol. 2007;37(3):176–185. doi: 10.1016/j.pediatrneurol.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Sass K.J., Sass A., Westerveld M., Lencz T., Novelly R.A., Kim J.H., Spencer D.D. Specificity in the correlation of verbal memory and hippocampal neuron loss: dissociation of memory, language, and verbal intellectual ability. J. Clin. Exp. Neuropsychol. 1992;14(5):662–672. doi: 10.1080/01688639208402854. [DOI] [PubMed] [Google Scholar]
- Sass K.J., Spencer D.D., Kim J.H., Westerveld M., Novelly R.A., Lencz T. Verbal memory impairment correlates with hippocampal pyramidal cell density. Neurology. 1990;40(11):1694–1697. doi: 10.1212/wnl.40.11.1694. [DOI] [PubMed] [Google Scholar]
- Slinger G., Sinke M.R., Braun K.P., Otte W.M. White matter abnormalities at a regional and voxel level in focal and generalized epilepsy: a systematic review and meta-analysis. Neuroimage Clin. 2016;12:902–909. doi: 10.1016/j.nicl.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.L. Rethinking cognition and behavior in the new classification for childhood epilepsy: examples from frontal lobe and temporal lobe epilepsies. Epilepsy Behav. 2016;64(Pt B):313–317. doi: 10.1016/j.yebeh.2016.04.050. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Beckmann C.F., Andersson J., Auerbach E.J., Bijsterbosch J., Douaud G., Consortium W.-M.H. Resting-state fMRI in the human connectome project. Neuroimage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O., Honey C.J., Kotter R. Identification and classification of hubs in brain networks. PLoS ONE. 2007;2(10) doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O., Benton A.L. revised ed. Neuropsychology Laboratory, University of Victoria; Victoria, BC, Canada: 1977. Neurosensory Center Comprehensive Examination for Aphasia: Manual of Directions. [Google Scholar]
- Steer S., Pickrell W.O., Kerr M.P., Thomas R.H. Epilepsy prevalence and socioeconomic deprivation in England. Epilepsia. 2014;55(10):1634–1641. doi: 10.1111/epi.12763. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Arenaza-Urquijo E.M., Bartres-Faz D., Belleville S., Cantilon M., Chetelat G., Conceptual Frameworks W. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2018 doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E., Sherman E., Spreen O. 3rd ed. Oxford University Press; New York: 2006. A Compendium of Neuropsychological Tests. [Google Scholar]
- Stretton J., Thompson P.J. Frontal lobe function in temporal lobe epilepsy. Epilepsy Res. 2012;98(1):1–13. doi: 10.1016/j.eplepsyres.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R., Walther G., Hastie T. Estimating the number of clusters in a data set via the gap statistic. J. R. Statist. Soc. Series B-Statist. Methodol. 2001;63:411–423. doi: 10.1111/1467-9868.00293. [DOI] [Google Scholar]
- Tiemeier H., Lenroot R.K., Greenstein D.K., Tran L., Pierson R., Giedd J.N. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010;49(1):63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenerry M.R., Jack C.R., Jr., Ivnik R.J., Sharbrough F.W., Cascino G.D., Hirschorn K.A., Meyer F.B. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43(9):1800–1805. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- van Diessen E., Zweiphenning W.J., Jansen F.E., Stam C.J., Braun K.P., Otte W.M. Brain network organization in focal epilepsy: a systematic review and meta-analysis. PLoS ONE. 2014;9(12) doi: 10.1371/journal.pone.0114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loenhoud A.C., Groot C., Vogel J.W., van der Flier W.M., Ossenkoppele R. Is intracranial volume a suitable proxy for brain reserve? Alzheimers Res. Ther. 2018;10(1):91. doi: 10.1186/s13195-018-0408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendramin L., Campello R.J.G.B., Hruschka E.R. Relative clustering validity criteria: a comparative overview. Stat. Anal. Data Min. 2010;3:209–235. doi: 10.1002/sam.10080. [DOI] [Google Scholar]
- Verche E., San Luis C., Hernandez S. Neuropsychology of frontal lobe epilepsy in children and adults: systematic review and meta-analysis. Epilepsy Behav. 2018;88:15–20. doi: 10.1016/j.yebeh.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Verrotti A., Matricardi S., Rinaldi V.E., Prezioso G., Coppola G. Neuropsychological impairment in childhood absence epilepsy: review of the literature. J. Neurol. Sci. 2015;359(1–2):59–66. doi: 10.1016/j.jns.2015.10.035. [DOI] [PubMed] [Google Scholar]
- Wandschneider B., Thompson P.J., Vollmar C., Koepp M.J. Frontal lobe function and structure in juvenile myoclonic epilepsy: a comprehensive review of neuropsychological and imaging data. Epilepsia. 2012;53(12):2091–2098. doi: 10.1111/epi.12003. [DOI] [PubMed] [Google Scholar]
- Wang J., Zuo X., He Y. Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 2010;4:16. doi: 10.3389/fnsys.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.H., Liou H.H., Chen C.C., Chiu M.J., Chen T.F., Cheng T.W., Hua M.S. Neuropsychological performance and seizure-related risk factors in patients with temporal lobe epilepsy: a retrospective cross-sectional study. Epilepsy Behav. 2011;22(4):728–734. doi: 10.1016/j.yebeh.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Wechsler D. NCS Pearson; San Antonio, TX: 2011. Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II) [Google Scholar]
- Whelan C.D., Altmann A., Botia J.A., Jahanshad N., Hibar D.P., Absil J., Sisodiya S.M. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain. 2018;141(2):391–408. doi: 10.1093/brain/awx341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens S., Bowden S.C., D'Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: a systematic review and meta-analysis. Epilepsia. 2017;58(10):1673–1685. doi: 10.1111/epi.13865. [DOI] [PubMed] [Google Scholar]
- Williams J.C., Van Snellenberg J.X. Motion denoising of multiband resting state functional connectivity MRI data: an improved volume censoring method. bioRxiv. 2019 doi: 10.1101/860635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.J., Baxendale S. The new approach to classification: rethinking cognition and behavior in epilepsy. Epilepsy Behav. 2014;41:307–310. doi: 10.1016/j.yebeh.2014.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regional analyses of cortical volume and thickness.
Correcting for age and sex.
Correcting for age and laterality.
Nodes used in graph theory.
Data Availability Statement
Efforts are ongoing to release raw DICOM (digital imaging and communications in medicine) data from the ECP through the CCF (Connectome Coordination Facility, www.humanconnectome.org/software/connectomedb) (Hodge et al., 2016) at Washington University in St. Louis.