Abstract
Changes in volatile compounds of fermented minced pepper (FMP) during natural fermentation (NF) and inoculated fermentation (IF) process were analyzed by the headspace–gas chromatography–ion mobility spectrometry (HS‐GC‐IMS). A total of 53 volatile compounds were identified, including 12 esters, 17 aldehydes, 13 alcohols, four ketones, three furans, two acids, one pyrazine, and one ether. Generally, fermentation time played an important role in volatile compounds of FMP. It was found that most esters, aldehydes, and alcohols obviously decreased with the increase in fermentation time, including isoamyl hexanoate, methyl octanoate, gamma‐butyrolactone, phenylacetaldehyde, methional, and E‐2‐hexenol. Only a few volatile compounds increased, especially for 2‐methylbutanoic acid, 2‐methylpropionic acid, linalool, ethanol, and ethyl acetate. However, no significant difference in volatile compounds was found between NF and IF samples at the same fermentation time. In addition, the fermentation process in all samples was well discriminated as three stages (0 days; 6 day; and 12, 18, and 24 days), and all volatile compounds were divided into two categories (increase and decrease) based on principal component analysis and heat map.
Keywords: fermentation process, fermented minced pepper, headspace–gas chromatography–ion mobility spectrometry, volatile compounds
Changes in volatile compounds of FMP during NF and IF process were analyzed by HS‐GC‐IMS. Fermentation time played an important role in volatile compounds of FMP. Most esters, aldehydes, and alcohols obviously decreased, and only a few volatile compounds increased during NF and IF, especially for acids. NF and IF posed little effect on volatile compounds of FMP.

1. INTRODUCTION
Pepper (Capsicum annuum L.), belonging to the Solanaceae family, is widely planted worldwide and covers approximately 1.99 million ha of harvested area and an annual production of 36.77 million tons in 2018 according to FAO (2018). Pepper fruits are rich in nutrients and bioactive compounds, including L‐ascorbic acid, phenolic compounds, carotenoids, and capsaicin, which exhibit antioxidant activities and anti‐inflammatory effects (Hernández‐Carrión et al., 2015; Ribes‐Moya, Raigón, Moreno‐Peris, Fita, & Rodríguez‐Burruezo, 2018). Nowadays, pepper fruits are an important ingredient in several fermented food, including kimchi, fermented pepper paste, and fermented minced pepper (FMP; Li et al., 2019; Wang, Wang, Xiao, Liu, Deng, et al., 2019; Wang, Wang, Xiao, Liu, Jiang, et al., 2019). FMP, as a traditional and local fermented vegetable in the southern regions of China, is widely consumed due to its nutritional and sensory properties (Li, Zhao, et al., 2016). It can be eaten directly or used as cooking ingredient. FMP can be prepared by natural fermentation (NF) and inoculated fermentation (IF). NF was a kind of fermentation methods in which microorganisms from natural environment were used for fermentation, and IF was a kind of fermentation methods in which inoculated microorganisms were used for fermentation. Whatever NF or IF, lactic acid bacteria (LAB) become the dominant microorganism when conditions are suitable for their growth (Sanlier, Gökcen, & Sezgin, 2017). Meanwhile, LAB fermentation involves the production of various metabolites in FMP such as alcohols, organic acids, and active metabolites that contribute to its nutrition, taste, flavor, and functionality (Wang, Wang, Xiao, Liu, Deng, et al., 2019; Wang, Wang, Xiao, Liu, Jiang, et al., 2019).
Flavor plays a key important role in defining sensory and consumer acceptance of FMP. The flavor of FMP is a complex trait, including hot taste from peppers, umami taste from amino acids, and salty taste from NaCl. Previous researchers have focused on flavor characteristics of fermented vegetables products, including fermented peppers, sauerkraut, and kimchi (Sanlier et al., 2017). Wang, Wang, Xiao, Liu, Deng, et al. (2019) showed that alcohols, esters, and ketones were the dominant volatile fractions in fermented/chopped pepper by solid‐phase microextraction and gas chromatography–mass spectrometry, and the fermentation stage was mainly affected by esters, alcohols, aldehydes, and terpenes. Liu et al. (2019) found that the flavor profiles of Sichuan pickle fermented in glass jars (GL), porcelain jars (PO), and plastic jars (PL) were different. The compound with the highest concentration in both PO and GL was the alkanes, while the highest concentration of compound was ester in the PL. Wu et al. (2015) measured changes of flavor compounds in suan cai during NF, and found that there were 17 varieties of volatile flavor components in the early fermentation time, but increased to 57 in the middle fermentation time. In addition, the result also showed that esters and aldehydes were in the greatest diversity and abundance, contributed most to the aroma of suan cai. Kang and Baek (2014) found that 19 aroma‐active compounds were detected by aroma extract dilution analysis in Korean fermented red pepper paste (gochujang), and 12 aroma‐active compounds were detected by headspace–solid‐phase microextraction–gas chromatography–olfactometry. Hence, the variety and content of volatile compounds in fermented vegetables could be affected by multiple factors, such as raw material, container, and fermentation time. For FMP, the previous research and industrial production mainly focused on the optimization of process parameters, including raw material, NaCl and CaCl2 content, LAB inoculum, fermentation temperature, and time based on the change of quality. However, knowledge about changes in flavor compounds of FMP at different stages with NF or IF is not available.
Ion mobility spectrometry (IMS), a rapid detection technique, was used to detect the gasified volatile compounds by ion separation based on their ion mobility velocity (Zhang et al., 2016). The detection technology presented many advantages, including easy operation, high analysis speed, high sensitivity, and no complex sample preparation steps. However, its analysis characteristics were often limited for complex samples, especially for complex systems in food and agricultural products (Arce et al., 2014). Combining IMS with other instruments is a more suitable and effective way to make better use of its advantages. Recently, headspace–gas chromatography–ion mobility spectrometry (HS‐GC‐IMS) has been applied in detecting volatile compounds of fruits and vegetables, such as candied kumquats (Hu et al., 2019), jujube fruits (Yang et al., 2019), and dried peppers (Ge et al., 2020). As a consequence, HS‐GC‐IMS is effective to identify the flavor characteristic of fruits and vegetables. However, the changes in characteristic compounds linked to the flavors of FMP during NF and IF process are still not available. Hence, HS‐GC‐IMS can be used to establish fingerprints of volatile compounds in FMP during NF and IF process.
In this study, the changes of volatile compounds in FMP during NF and IF process were analyzed, and several target volatile compounds in samples were detected using HS‐GC‐IMS, principal component analysis (PCA), and the heat map. The results confirmed the potential of HS‐GC‐IMS to identify the volatile compound characteristics and provided a rapid method to determine the flavor quality of FMP during fermentation process.
2. MATERIALS AND METHODS
2.1. Materials
Fresh peppers (Capsicum annuum L.) cv. “yanhong” were grown in an experimental field of Gaoqiao Town, Changsha County, Hunan Province, China (N28°28′38.08, E113°20′54.65″). All harvested peppers were up to commercial maturity. After harvesting, the peppers were delivered to the laboratory immediately. Peppers with uniformity of size, color, and weight, free from visible blemishes, disease, and/or physical damage, were selected as the experiment raw materials. W‐4 LAB was isolated, purified, and extended culture from our previous FMP obtained by NF, and it posed strong resistance to high salt and acid.
2.2. Preparation of FMP
Selected peppers were removed handle, washed, drained, and minced into small pieces (0.5–1 cm × 0.5–1 cm), and 10% (w/w) NaCl and 0.1% CaCl2 (w/w) were added to the minced peppers and then stirred for 5 min. Above samples were divided into two groups: One group inoculated with 5% (w/w) W‐4 LAB (107 CFU/ml) was regarded as IF, and another group without inoculated LAB was regarded as NF. Prepared samples were put into pickle jars, and the jars were sealed with water to exclude air and fermented in a 30°C incubator. FMPs were obtained on 0, 6, 12, 18, and 24 days, respectively. The collected samples were detected immediately after freeze‐drying and grinding.
2.3. HS‐GC‐IMS analysis
All the analyses were obtained by HS‐GC‐IMS instrument and related supplementary analysis software according to the method of Sun et al. (2019) with some modifications. Freeze‐dried (0.5 g) FMP was weighted and then transferred into a 20‐ml headspace bottle. The FMP was incubated at 80°C in the headspace bottle, with the speed of 500 rpm for 10 min. After incubation, 500 μl headspace was automatically injected using a heated syringe at 85°C into a FS‐SE‐54‐CB‐1 (15 m × 0.53 mm ID) capillary column. The carried gas during injection was nitrogen (99.99% purity), which was under the below programmed flow to carry samples: 2 ml/min held for 2 min, flowed ramp from 2 ml/min to 100 ml/min in 18 min, and then maintained 100 ml/min for 10 min until stopping. The analyses were separated in the column at 60°C and then ionized in the IMS ionization chamber at 45°C. The constant flow of drift gas flow was set up to 150 ml/min. The instrument was standardized by linear retention index (RI) of n‐ketones, for the reason that IMS had no response to alkanes. Calculation of RI of volatile compounds was based on the n‐ketones C4‐C9. Comparing the drift time and RI in the GC‐IMS library, volatile compounds in NF and IF samples were well identified. The qualitative analysis of volatile compounds was conducted based on the IMS and NIST database built in GC × IMS Library Search. The quantitative analysis for volatile compounds was mainly based on the peak intensity in HS‐GC‐IMS, and the peak intensity was proportional to the content of volatile compounds.
2.4. Data analysis
All the experiment was performed in triplicate. The spectra were analyzed with laboratory analytical viewer (LAV), and the difference profiles and fingerprints of volatile compounds were constructed with the Reporter and Gallery plug‐ins. The NIST and IMS database were built into the software for qualitative analysis of samples. The line and bar charts drawn by Origin 2018 were used to analyze change of volatile compounds in FMP during fermentation process. PCA obtained by the original date was used for clustering analysis of principal compounds in samples. The heat map was generated using the heat map plug‐in of origin 2018, and the methods used for clustering of original date were Ward minimum variance and Euclidean distance.
3. RESULTS AND DISCUSSION
3.1. Changes of HS‐GC‐IMS spectra in FMP
The volatile compounds of FMP during NF and IF were detected by HS‐GC‐IMS. The data were exhibited with the 3D spectrum, where the x‐axis represented the ion migration time, the y‐axis represented the retention time of the gas chromatograph, and the z‐axis represented the peak intensity. As shown in Figure 1, there was the similar peak signal distribution in all samples during fermentation process. This phenomenon suggested that all FMP posed the same volatile compounds during fermentation process. However, the peak signal intensity showed some differences in FMP during fermentation process. It indicated that content of volatile compounds changed (increasing or decreasing) with the prolonging of fermentation time. Yu et al. (2013) proved that the prominent microorganisms posed clear relationships with changes in flavor during fermentation. However, no obvious differences in volatile compounds were found between NF and IF samples. It was found that the signal intensities of almost all volatile compounds in IF samples were similar to those in NF ones. This phenomenon showed that two fermentation methods played minor effects on changes in volatile compounds during fermentation process.
FIGURE 1.
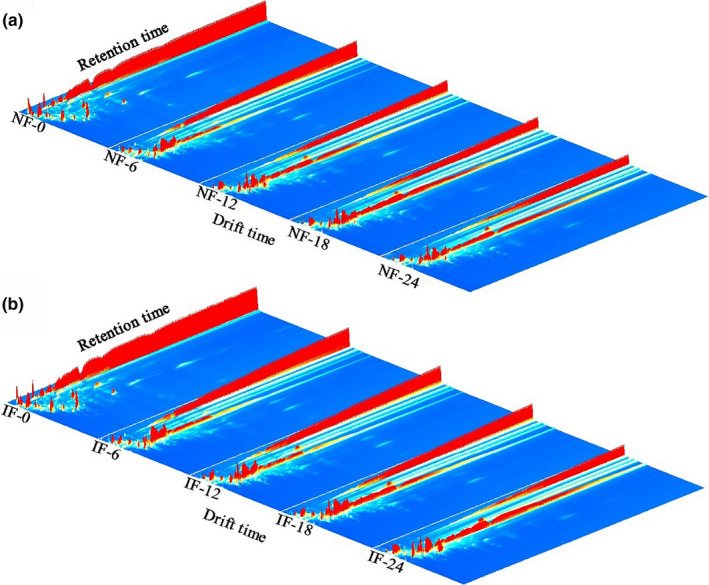
Changes in 3D topography of volatile compounds during NF and IF process. (a) NF; (b) IF. IF: inoculated fermentation; NF: natural fermentation
The volatile compounds in FMP were not easy for analysis by 3D spectra. Hence, the 2D spectra were used for further comparison, as shown in Figure 2. The reactive ion peak (RIP) was exhibited with the red vertical line at the horizontal coordinate of 1.0. The normalized migration time, from 7.92 to 7.97 ms, was used to avoid the change of ion migration time caused by temperature and pressure deviation during detection process. For comparing with the differences in volatile compounds in all samples, fresh samples were taken as the reference, and the spectral background colors of other samples were white after deducting that of original samples. In 2D spectra, every dot on the right side of RIP represented the specific volatile compound. The color of dots represented the concentration of volatile compounds. The blue dots represented that the volatile compounds posed lower concentration comparing with those of the control, and the red dots represented that the volatile compounds posed higher concentration comparing with those of the control. As shown in Figure 2a,b, most dots were located in 2D spectra area from 0 to 400 s of retention time and from 1.0 to 1.5 of drift time, and few dots were located in 2D spectra area from 400 to 1,200 s of retention time.
FIGURE 2.
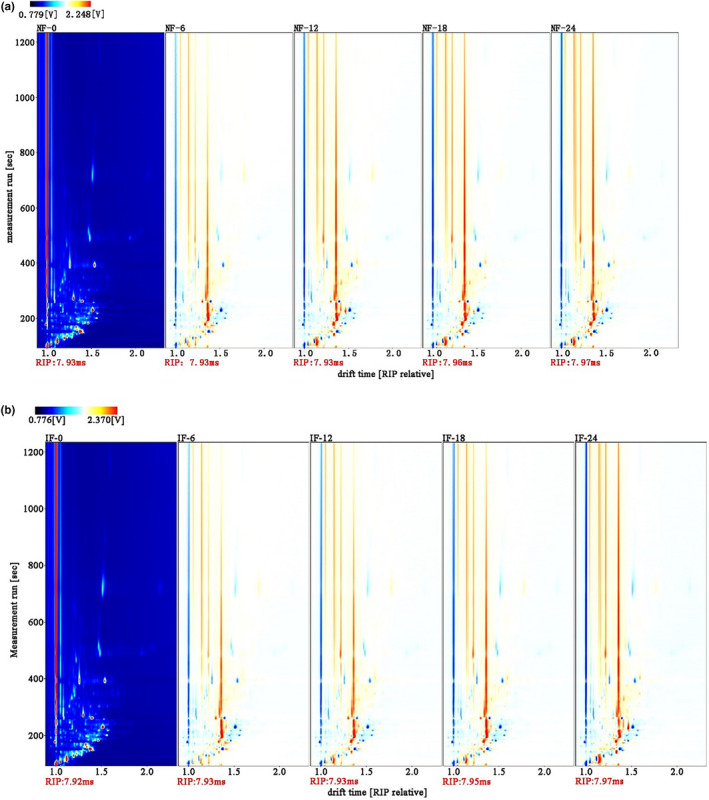
Changes in 2D topography of volatile compounds during NF and IF process. (a) NF; (b) IF. IF: inoculated fermentation; NF: natural fermentation
As shown in Figure 2a,b, the total number of dots in 2D spectra hardly changed. However, the number of blue or red dots changed (increase or decrease). These results indicated that the varieties of volatile compounds in samples were the same, but the content of volatile compounds changed with the prolonging of fermentation time. The reason was that metabolism and decomposition of microorganisms caused volatile compounds to increase or decrease during fermentation process. Wu et al. (2015) showed that various bacteria and fungi, especially for LAB and yeast, possessed obvious correlation with the changes in volatile compounds during fermentation process. In addition, some pathways of biosynthesis and degradation also existed in FMP, including EMP pathway, Strecker pathway, and decarboxylation pathway, which could increase volatile compound content (Kang & Baek, 2014; Li, Zhao, et al., 2016; Li, Dong, Huang, & Wang, 2016). However, the color of red or blue dots in NF and IF samples was similar to each other. Therefore, the content of volatile compounds in NF and IF samples was almost the same. This result was because of the fact that LAB was the prominent microorganism under suitable conditions whatever NF or IF.
3.2. Qualitative analysis of volatile compounds in FMP
The qualitative analysis of volatile compounds in FMP during fermentation process was represented by numbers, as shown in Figure 3 and Table 1. Each dot represented a type of volatile compound. The marked dots were identified volatile compounds, but unmarked dots were nonidentified volatile compounds. All FMP showed 53 identified dots by GC × IMS library analysis. Therefore, there were the same volatile compounds in all samples. This result indicated that fermentation methods hardly affected varieties of volatile compounds during fermentation process. In Figure 3 and Table 1, a total of 43 typical volatile compounds, including nine esters, 12 aldehydes, 11 alcohols, four ketones, three furans, two acids, one pyrazine, and one ether, were identified by NIST and IMS database in all samples. However, monomer and dimer of the same volatile compound exhibited similar retention time, but different migration time. The volatile compounds with high content or high proton affinity were beneficial for the production of new dimer (Arroyo‐Manzanaresa et al., 2018; Lantsuzskaya, Krisilov, & Levina, 2015). Arroyo‐Manzanaresa et al. (2018) found that several monomers with proton affinity absorbed some reactant protons, and then formed dimer by the combination of different monomers. Meanwhile, several volatile compounds could produce different signals, consisting of isoamyl hexanoate, methyl octanoate, nonanal, phenylacetaldehyde, heptadienal, benzaldehyde, gamma‐butyrolactone, methional, E‐2‐hexenol, hexanal, methylbutanal, methylbutanol, and ethanol. Li et al. (2010) showed that the same volatile compounds with different concentrations also might produce multiple signals in tricholoma matsutake. In addition, new formed dimer showed higher molecular weight than the monomer, and further generated multiple signals.
FIGURE 3.
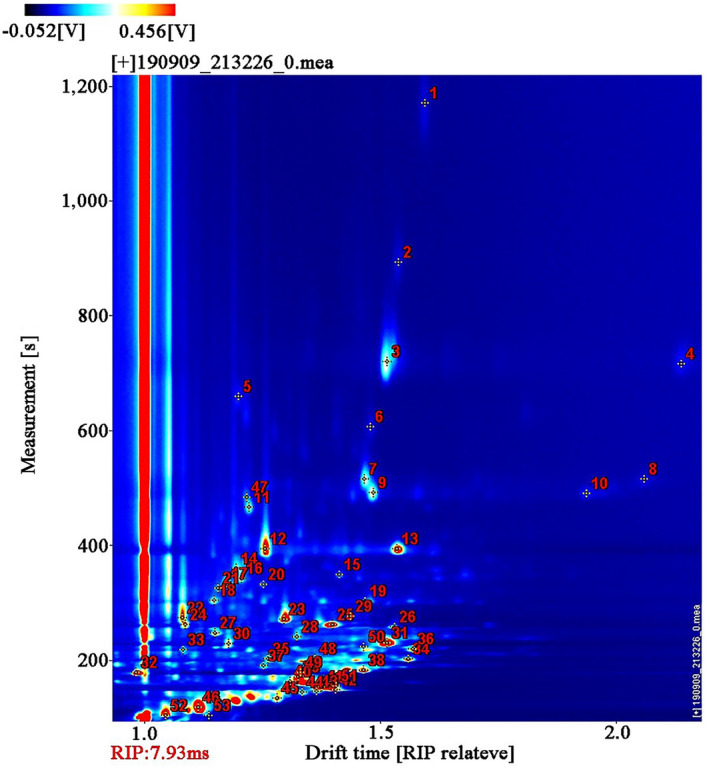
Ion migration spectra of volatile compounds identified by HS‐GC‐IMS during NF and IF process. IF: inoculated fermentation; NF: natural fermentation
TABLE 1.
Information of identified volatile compounds during NF and IF process
| Count | Compound | CAS# | Formula | MW | RI | Rt (s) | Dt (RIPrel) | Comment | Identification |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Hexyl hexanoate | C6378650 | C12H24O2 | 200.3 | 1,558.7 | 1,170.184 | 1.5963 | RI,Dt | |
| 2 | Ethyl nonanoate | C123295 | C11H22O2 | 186.3 | 1,374.8 | 892.49 | 1.5408 | RI,Dt | |
| 3 | Isoamyl hexanoate | C2198610 | C11H22O2 | 186.3 | 1,260.8 | 720.352 | 1.5153 | Monomer | RI,Dt |
| 4 | Isoamyl hexanoate | C2198610 | C11H22O2 | 186.3 | 1,257.5 | 715.481 | 2.1399 | Dimer | RI,Dt |
| 5 | Methyl salicylate | C119368 | C8H8O3 | 152.1 | 1,220.3 | 659.302 | 1.2006 | RI,Dt | |
| 6 | Ethyl octanoate | C106321 | C10H20O2 | 172.3 | 1,185.2 | 606.274 | 1.4812 | RI,Dt | |
| 7 | Methyl octanoate | C111115 | C9H18O2 | 158.2 | 1,124.9 | 515.216 | 1.468 | Monomer | RI,Dt |
| 8 | Methyl octanoate | C111115 | C9H18O2 | 158.2 | 1,124.5 | 514.614 | 2.0618 | Dimer | RI,Dt |
| 9 | Nonanal | C124196 | C9H18O | 142.2 | 1,108.9 | 491.123 | 1.4867 | Monomer | RI,Dt |
| 10 | Nonanal | C124196 | C9H18O | 142.2 | 1,108.5 | 490.521 | 1.9397 | Dimer | RI,Dt |
| 11 | Methyl benzoate | C93583 | C8H8O2 | 136.1 | 1,092.5 | 466.428 | 1.2237 | RI,Dt | |
| 12 | Phenylacetaldehyde | C122781 | C8H8O | 120.2 | 1,043.3 | 394.148 | 1.2576 | Monomer | RI,Dt |
| 13 | Phenylacetaldehyde | C122781 | C8H8O | 120.2 | 1,042.9 | 393.546 | 1.5376 | Dimer | RI,Dt |
| 14 | E‐E‐2‐4‐Heptadienal | C4313035 | C7H10O | 110.2 | 1,016.8 | 358.741 | 1.196 | RI,Dt | |
| 15 | Octanal | C124130 | C8H16O | 128.2 | 1,008.3 | 348.385 | 1.4139 | RI,Dt | |
| 16 | E‐Z‐2‐4‐Heptadienal | C4313024 | C7H10O | 110.2 | 1,002.6 | 341.766 | 1.2064 | RI,Dt | |
| 17 | 6‐Methyl‐5‐hepten‐2‐one | C110930 | C8H14O | 126.2 | 995.3 | 333.382 | 1.1743 | RI,Dt | |
| 18 | Benzaldehyde | C100527 | C7H6O | 106.1 | 965.2 | 303.377 | 1.1489 | Monomer | RI,Dt |
| 19 | Benzaldehyde | C100527 | C7H6O | 106.1 | 964.7 | 302.936 | 1.4701 | Dimer | RI,Dt |
| 20 | 2‐Pentylfuran | C3777693 | C9H14O | 138.2 | 994.0 | 332.058 | 1.2533 | RI,Dt | |
| 21 | 1‐Octen‐3‐ol | C3391864 | C8H16O | 128.2 | 987.5 | 324.998 | 1.1569 | RI,Dt | |
| 22 | Gamma‐butyrolactone | C96480 | C4H6O2 | 86.1 | 927.0 | 272.937 | 1.0809 | Monomer | RI,Dt |
| 23 | Gamma‐butyrolactone | C96480 | C4H6O2 | 86.1 | 924.0 | 270.819 | 1.2971 | Dimer | RI,Dt |
| 24 | Methional | C3268493 | C4H8OS | 104.2 | 909.5 | 261.135 | 1.0882 | Monomer | RI,Dt |
| 25 | Methional | C3268493 | C4H8OS | 104.2 | 910.5 | 261.74 | 1.4003 | Dimer | RI,Dt |
| 26 | 2‐6‐Dimethylpyrazine | C108509 | C6H8N2 | 108.1 | 904.4 | 257.806 | 1.5315 | RI,Dt | |
| 27 | Cyclohexanone | C108941 | C6H10O | 98.1 | 887.1 | 247.358 | 1.1514 | RI,Dt | |
| 28 | 1‐Hexanol | C111273 | C6H14O | 102.2 | 876.0 | 241.002 | 1.3241 | RI,Dt | |
| 29 | 2‐Acetylfuran | C1192627 | C6H6O2 | 110.1 | 932.1 | 276.569 | 1.4392 | RI,Dt | |
| 30 | E‐2‐Hexenol | C928950 | C6H12O | 100.2 | 852.7 | 228.451 | 1.1793 | Monomer | RI,Dt |
| 31 | E‐2‐Hexenol | C928950 | C6H12O | 100.2 | 855.0 | 229.661 | 1.5157 | Dimer | RI,Dt |
| 32 | Dimethyl disulfide | C624920 | C2H6S2 | 94.2 | 743.1 | 178.214 | 0.9837 | RI,Dt | |
| 33 | Furfurol | C98011 | C5H4O2 | 96.1 | 831.3 | 217.693 | 1.0831 | RI,Dt | |
| 34 | Hexanal | C66251 | C6H12O | 100.2 | 796.8 | 201.459 | 1.5618 | Dimer | RI,Dt |
| 35 | Hexanal | C66251 | C6H12O | 100.2 | 797.2 | 201.665 | 1.2638 | Monomer | RI,Dt |
| 36 | 3‐Methylpentanol | C589355 | C6H14O | 102.2 | 833.4 | 218.721 | 1.5706 | RI,Dt | |
| 37 | 1‐Pentanol | C71410 | C5H12O | 88.1 | 770.0 | 189.54 | 1.2531 | RI,Dt | |
| 38 | 2‐Methylbutanol | C137326 | C5H12O | 88.1 | 754.2 | 182.759 | 1.4661 | RI,Dt | |
| 39 | Acetoin | C513860 | C4H8O2 | 88.1 | 722.3 | 170.019 | 1.3273 | RI,Dt | |
| 40 | 2‐Ethylfuran | C3208160 | C6H8O | 96.1 | 699.3 | 161.799 | 1.3107 | RI,Dt | |
| 41 | 1‐Butanol | C71363 | C4H10O | 74.1 | 671.5 | 153.168 | 1.3781 | RI,Dt | |
| 42 | 3‐Methylbutanal | C590863 | C5H10O | 86.1 | 653.7 | 148.27 | 1.4073 | RI,Dt | |
| 43 | 2‐Methylpropanol | C78831 | C4H10O | 74.1 | 642.2 | 145.31 | 1.3662 | RI,Dt | |
| 44 | Ethyl acetate | C141786 | C4H8O2 | 88.1 | 637.6 | 144.153 | 1.3364 | RI,Dt | |
| 45 | Butanal | C123728 | C4H8O | 72.1 | 594.7 | 133.973 | 1.283 | RI,Dt | |
| 46 | Acetone | C67641 | C3H6O | 58.1 | 524.9 | 117.893 | 1.1158 | RI,Dt | |
| 47 | Linalool | C78706 | C10H18O | 154.3 | 1,103.6 | 483.186 | 1.218 | RI,Dt | |
| 48 | 2‐Methylpropionic acid | C79312 | C4H8O2 | 88.1 | 798.3 | 202.172 | 1.363 | RI,Dt | |
| 49 | 3‐Methylbutanol | C123513 | C5H12O | 88.1 | 744.5 | 178.74 | 1.3325 | RI,Dt | |
| 50 | 2‐Methylbutanoic acid | C116530 | C5H10O2 | 102.1 | 843.4 | 223.707 | 1.4662 | RI,Dt | |
| 51 | 2‐Methylbutanal | C96173 | C5H10O | 86.1 | 675.7 | 154.407 | 1.4087 | RI,Dt | |
| 52 | Ethanol | C64175 | C2H6O | 46.1 | 458.1 | 102.494 | 1.0455 | Monomer | RI,Dt |
| 53 | Ethanol | C64175 | C2H6O | 46.1 | 461.2 | 103.211 | 1.1372 | Dimer | RI,Dt |
3.3. Fingerprint analysis of volatile compounds in FMP
Although the 3D and 2D spectrum presented the change tendency of volatile compounds during NF and IF, the specific volatile compounds were not accurately judged. Hence, all signal peaks were used to make a detailed fingerprint analysis, as shown in Figure 4. In fingerprints, each column represented a kind of volatile compounds, each row represented the FMP samples, and volatile compound content was determined by the brightness degree of color.
FIGURE 4.
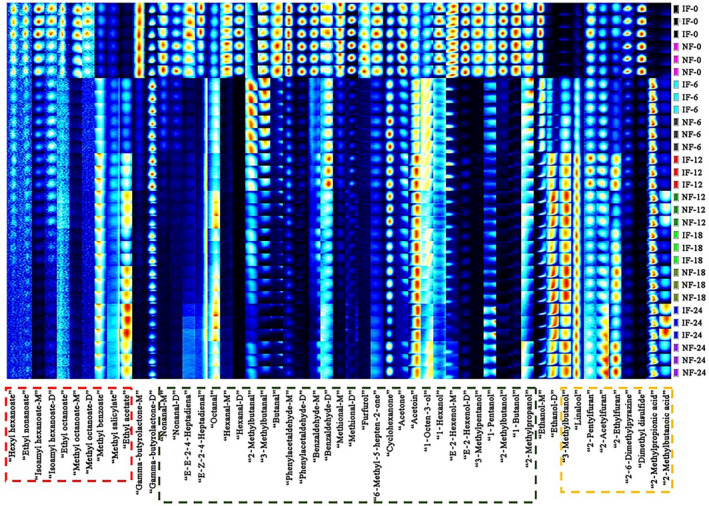
Changes in fingerprint of volatile compounds during NF and IF process. D: dimer; IF: inoculated fermentation; IF‐0, IF‐6, IF‐12, IF‐18, IF‐24: IF samples were obtained on 0, 6, 12, 18, and 24 days; M: monomer; NF: natural fermentation; NF‐0, NF‐6, NF‐12, NF‐18, NF‐24: NF samples were obtained on 0, 6, 12, 18, and 24 days
In the raw materials (fermented on 0 days), high content of esters, aldehydes, and alcohols were found, especially for isoamyl hexanoate, gamma‐butyrolactone, phenylacetaldehyde, methional, E‐2‐hexanol, and 1‐butanol. The change in volatile compounds during NF and IF was presented in different color frames. In the red frame, the content of volatile compounds, such as hexyl hexanoate, ethyl nonanoate, isoamyl hexanoate, ethyl octanoate, and methyl octanoate, decreased to a low level. The decrease in above volatile compounds was related to the accumulation of acids. High acid condition induced by microorganisms could accelerate the hydrolysis of some volatile compounds (Bautista‐Expósito, Peñas, Silván, Frias, & Martínez‐Villaluenga, 2018; Tomita, Nakamura, & Okada, 2018). In addition, the content of several volatile compounds increased to a high level, such as ethyl acetate. However, the fingerprint brightness of above volatile compounds in NF samples was similar to those in IF samples during fermentation process. In the green frame, many volatile compounds sharply decreased with the prolonging of fermentation time, and showed low signal intensity and gray color in the late fermentation time. These volatile compounds were mainly aldehydes (nonanal, E‐Z‐2‐4‐heptadienal, hexanal, methylbutanal, etc.), ketones (acetone, acetoin, etc.), and alcohols (methylpentanol, 1‐butanol, etc.). This result was slightly different from the results of Wang, Wang, Xiao, Liu, Deng, et al. (2019), which might be related to pepper species, fermented methods, and the concentration of salt. Otherwise, the content of above volatile compounds showed similar fingerprint between NF and IF samples. In the yellow frame, the brightness degree of color in alcohols (linalool), furans (2‐ethylfuran, 2‐acetylfuran), and acids (2‐methylpropionic acid, 2‐methylbutanoic acid) increased with the prolonging of fermentation time, which indicated that these volatile compound content increased to a maximal level at the end of NF or IF. However, these volatile compounds in IF samples posed similar content with those in NF samples during fermentation process. Above result was accordance with the 3D and 2D spectra analysis. Moreover, the content of 2‐pentylfuran showed a fluctuate trend. To compare with volatile compound content in NF and IF samples during fermentation process, peak intensity values about volatile compounds needed further exploration.
3.4. Changes in volatile compounds of FMP
Seven kinds of volatile compounds, including eight esters, eight aldehydes, eight alcohols, and 11 other volatile compounds (four ketones, three furans, two acids, one ether, and one pyrazine), were used to explore the variation during NF and IF. Vegetable fermentation could be divided into aerobic fermentation and anaerobic fermentation (Gobbetti, Di Cagno, & De Angelis, 2010). The production of FMP was mainly anaerobic fermentation, such as alcohol and lactic acid. As shown in Figure 5a–d and Table 2, most of volatile compound content decreased with prolonging of fermentation time, only several volatile compounds increased. The reason for this phenomenon was that the growth of many microorganisms in FMP was inhibited by high concentration of salt, low pH, and oxygen‐deficient environment, so reduced the development of many volatile compounds. However, LAB posed a strong ability to resist adverse environment and produced volatile compounds by fermentation (homo‐ and heterofermentative LAB fermentation), especially for organic acids and alcohols (Esteban‐Torres et al., 2015; Yu et al., 2013). The followed analysis was used to further explore the specific change in various types of volatile compounds.
FIGURE 5.
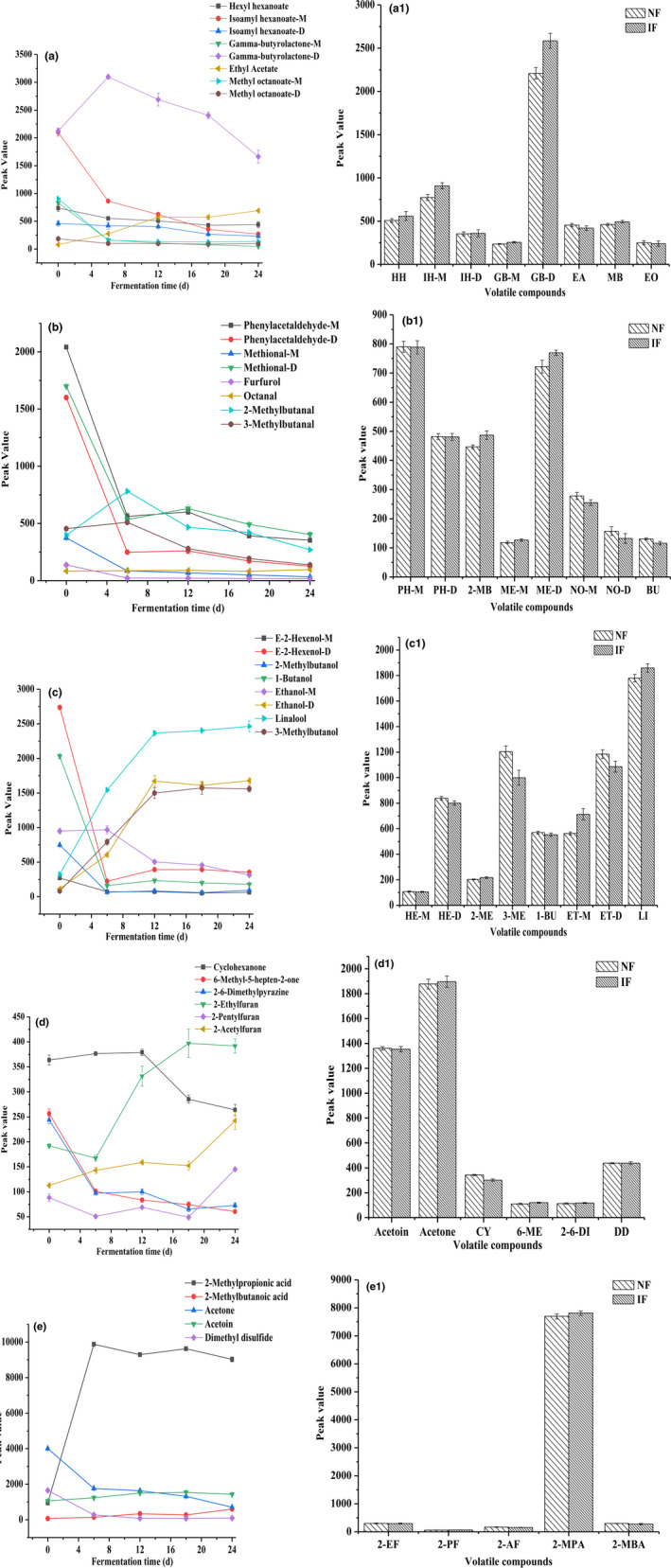
Changes in peak volume of volatile compounds during NF and IF process. NF: natural fermentation; IF: inoculated fermentation; (a, a1) esters; (b, b1) aldehydes; (c, c1) alcohols; (d, d1, e, e1) other volatile compounds (ketones, furans, acids, pyrazine, and ether); a, b, c, d, and e showed changes in peak volume of volatile compounds during NF and IF process; a1, b1, c1, d1, and e1 showed peak volume differences in volatile compounds between NF and IF samples. 1‐BU: 1‐butanol; 2‐6‐DI: 2‐6‐dimethylpyrazine; 2‐AF: 2‐acetylfuran; 2‐EF: 2‐ethylfuran; 2‐MBA: 2‐methylbutanoic acid; 2‐ME: 2‐methylbutanal; 2‐ME: 2‐methylbutanol; 2‐MPA: 2‐methylpropionic acid; 2‐PF: 2‐pentylfuran; 3‐ME: 3‐methylbutanol; 6‐ME: 6‐methyl‐5‐hepten‐2‐one; BU: butanal; CY: cyclohexanone; DD: dimethyl disulfide; EA: ethyl acetate; EO: ethyl octanoate; ET‐D: ethanol‐D; ET‐M: ethanol‐M; GB‐D: gamma‐butyrolactone‐D; GB‐M: gamma‐butyrolactone‐M; HE‐D: E‐2‐hexenol‐D; HE‐M: E‐2‐hexenol‐M; HH: hexyl hexanoate; IH‐D: isoamyl hexanoate‐D; IH‐M: isoamyl hexanoate‐M; LI: linalool; MB: methyl benzoate; ME‐D: methional‐D; ME‐M: methional‐M; NO‐D: nonanal‐D; NO‐M: nonanal‐M; PH‐D: phenylacetaldehyde‐D; PH‐M: phenylacetaldehyde‐M
TABLE 2.
The peak volume values of volatile compounds during NF and IF process
| Compound | NF | IF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 days | 6 days | 12 days | 18 days | 24 days | 0 days | 6 days | 12 days | 18 days | 24 days | |
| Esters | ||||||||||
| Hexyl hexanoate | 696.30 ± 20.34a | 559.14 ± 33.24b | 434.00 ± 12.77c | 408.59 ± 3.46c | 436.06 ± 41.10c | 779.21 ± 95.42a | 542.20 ± 28.10bc | 579.30 ± 51.81b | 448.62 ± 14.80c | 443.13 ± 69.57c |
| Ethyl nonanoate | 600.66 ± 41.68a | 407.66 ± 5.54b | 425.48 ± 24.07b | 387.19 ± 31.80b | 427.15 ± 85.05b | 628.67 ± 121.40a | 349.95 ± 96.91b | 423.90 ± 35.68b | 393.83 ± 64.64b | 406.79 ± 34.57b |
| Isoamyl hexanoate‐M | 1,891.90 ± 76.32a | 906.95 ± 24.72b | 502.06 ± 12.55c | 277.58 ± 37.58d | 289.29 ± 20.52d | 2,308.16 ± 63.08a | 817.42 ± 38.20b | 744.71 ± 31.23c | 430.00 ± 12.28d | 246.64 ± 27.50e |
| Isoamyl hexanoate‐D | 406.63 ± 21.23b | 477.36 ± 42.08a | 391.57 ± 30.30b | 242.91 ± 7.21c | 245.05 ± 12.14c | 515.36 ± 69.63a | 360.54 ± 77.93bc | 414.34 ± 11.48b | 291.81 ± 16.11cd | 216.06 ± 28.54d |
| Methyl salicylate | 184.20 ± 9.83b | 179.81 ± 13.57b | 213.58 ± 15.25a | 172.11 ± 21.31b | 182.27 ± 17.92b | 181.85 ± 24.87b | 166.66 ± 12.82b | 243.66 ± 6.86a | 190.73 ± 14.17b | 194.66 ± 17.17b |
| Ethyl octanoate | 309.48 ± 14.18a | 236.60 ± 18.51b | 243.39 ± 29.63b | 237.88 ± 19.48b | 224.92 ± 32.42b | 317.71 ± 48.60a | 204.26 ± 50.24b | 225.48 ± 23.26b | 225.25 ± 6.30b | 227.38 ± 23.10b |
| Methyl octanoate‐M | 832.48 ± 8.71a | 157.87 ± 6.11b | 131.33 ± 12.74cd | 117.48 ± 7.20d | 136.08 ± 12.27c | 977.04 ± 11.39a | 159.16 ± 17.56b | 133.20 ± 7.43b | 138.95 ± 18.19b | 138.54 ± 5.71b |
| Methyl octanoate‐D | 166.44 ± 10.03a | 108.47 ± 10.16b | 94.36 ± 1.57bc | 83.73 ± 2.62c | 100.26 ± 8.83b | 204.37 ± 40.59a | 111.87 ± 1.52b | 103.89 ± 2.62b | 90.41 ± 9.75b | 96.38 ± 17.43b |
| Gamma‐butyrolactone‐M | 825.36 ± 2.72a | 164.00 ± 2.65b | 75.09 ± 6.86c | 61.10 ± 3.34d | 54.93 ± 2.55d | 839.73 ± 18.28a | 160.32 ± 5.11b | 140.78 ± 11.56b | 91.15 ± 9.38c | 43.18 ± 1.68d |
| Gamma‐butyrolactone‐D | 2,129.95 ± 31.33b | 2,946.32 ± 19.31a | 2,071.85 ± 144.52b | 2,165.19 ± 82.49b | 1,725.34 ± 58.30c | 2,117.70 ± 70.64c | 3,250.77 ± 62.68a | 3,309.24 ± 86.57a | 2,646.14 ± 37.57b | 1,598.90 ± 174.77d |
| Methyl benzoate | 211.83 ± 7.43d | 421.04 ± 17.80c | 566.03 ± 23.24a | 570.57 ± 9.50a | 534.35 ± 9.30b | 276.18 ± 12.41c | 413.70 ± 5.22b | 575.99 ± 14.03a | 590.11 ± 33.00a | 608.06 ± 8.19a |
| Ethyl Acetate | 81.35 ± 2.18d | 238.55 ± 5.48c | 656.58 ± 52.67ab | 689.45 ± 32.42a | 606.97 ± 3.23b | 75.79 ± 5.42d | 306.21 ± 8.91c | 485.49 ± 39.70b | 455.22 ± 44.40b | 772.66 ± 31.42a |
| Aldehydes | ||||||||||
| Nonanal‐M | 901.72 ± 35.72a | 235.38 ± 6.27b | 99.42 ± 8.88c | 79.25 ± 1.18c | 75.29 ± 7.40c | 749.11 ± 13.39a | 256.23 ± 14.35b | 114.30 ± 6.70c | 87.70 ± 7.88d | 68.69 ± 7.37d |
| Nonanal‐D | 413.59 ± 41.93a | 130.30 ± 14.13b | 78.61 ± 4.86c | 79.17 ± 6.79c | 85.60 ± 6.99c | 277.11 ± 25.45a | 129.45 ± 15.12b | 91.67 ± 11.58c | 84.51 ± 16.67c | 79.35 ± 15.62c |
| Phenylacetaldehyde‐M | 2,049.89 ± 18.72a | 581.59 ± 14.96b | 533.98 ± 21.69c | 407.69 ± 28.34d | 377.50 ± 10.83d | 2,033.72 ± 20.30a | 541.22 ± 42.13c | 667.36 ± 20.72b | 374.30 ± 3.80d | 328.87 ± 25.21d |
| Phenylacetaldehyde‐D | 1,640.78 ± 5.51a | 253.47 ± 1.56b | 217.62 ± 26.72c | 160.80 ± 11.20d | 137.49 ± 5.31d | 1,559.10 ± 10.51a | 244.21 ± 9.30c | 302.99 ± 20.48b | 182.33 ± 8.99d | 116.76 ± 8.39e |
| E‐E‐2‐4‐Heptadienal | 166.42 ± 8.89a | 37.81 ± 2.19b | 36.48 ± 1.42b | 31.07 ± 5.03bc | 24.95 ± 2.46c | 234.56 ± 2.22a | 29.63 ± 7.76c | 42.18 ± 3.35b | 33.16 ± 2.43c | 24.95 ± 4.99c |
| E‐Z‐2‐4‐Heptadienal | 207.79 ± 18.55a | 153.22 ± 5.68b | 159.16 ± 7.36b | 156.15 ± 2.40b | 113.49 ± 7.36c | 286.46 ± 0.66a | 142.60 ± 4.82d | 192.44 ± 3.53b | 170.30 ± 13.49c | 138.35 ± 1.19d |
| Benzaldehyde‐M | 228.13 ± 4.24a | 114.96 ± 11.48b | 99.94 ± 3.19c | 95.88 ± 10.29c | 101.64 ± 4.45bc | 236.69 ± 11.75a | 100.88 ± 9.11c | 137.76 ± 8.25b | 143.83 ± 3.41b | 65.41 ± 2.06d |
| Benzaldehyde‐D | 109.47 ± 8.82a | 95.06 ± 4.54b | 64.24 ± 5.37c | 61.27 ± 2.75c | 57.42 ± 2.08c | 138.22 ± 3.63a | 85.08 ± 2.85c | 104.97 ± 6.17b | 74.21 ± 5.06d | 57.76 ± 3.35e |
| Methional‐M | 372.66 ± 4.09a | 90.70 ± 4.38b | 50.42 ± 2.66c | 40.37 ± 5.61d | 37.83 ± 5.15d | 374.08 ± 8.34a | 82.70 ± 2.29b | 86.40 ± 5.44b | 60.24 ± 2.73c | 30.72 ± 1.28d |
| Methional‐D | 1,745.87 ± 5.70a | 532.18 ± 8.10b | 503.26 ± 36.28b | 477.18 ± 26.30c | 351.82 ± 32.74d | 1,652.81 ± 11.12a | 536.69 ± 4.06c | 697.63 ± 21.16b | 506.88 ± 2.91d | 454.11 ± 6.13e |
| Furfurol | 151.51 ± 4.35a | 24.53 ± 1.35b | 21.30 ± 2.53bc | 20.33 ± 2.50bc | 19.44 ± 6.30c | 124.95 ± 7.69a | 21.36 ± 4.67b | 23.43 ± 2.09b | 18.71 ± 1.83b | 18.68 ± 4.29b |
| Hexanal‐M | 157.50 ± 4.51a | 20.09 ± 1.86b | 19.06 ± 1.90b | 17.76 ± 1.49b | 16.75 ± 1.68b | 139.62 ± 2.30a | 19.68 ± 4.41b | 22.23 ± 2.61b | 20.10 ± 1.77b | 17.51 ± 0.66b |
| Hexanal‐D | 480.58 ± 36.06a | 19.36 ± 2.35b | 13.80 ± 1.57b | 16.27 ± 1.63b | 17.79 ± 3.39b | 537.34 ± 63.31a | 15.68 ± 0.013b | 15.88 ± 3.34b | 17.27 ± 2.20b | 20.44 ± 4.49b |
| Butanal | 385.21 ± 3.31a | 72.40 ± 2.48b | 72.51 ± 0.81b | 58.45 ± 3.46c | 65.88 ± 6.31b | 350.60 ± 10.21a | 70.63 ± 5.74b | 63.33 ± 6.08b | 33.20 ± 1.02c | 62.34 ± 6.78b |
| Octanal | 87.73 ± 6.93b | 90.67 ± 1.82b | 114.84 ± 13.25a | 97.02 ± 11.21b | 81.40 ± 2.17b | 81.33 ± 6.15b | 85.09 ± 4.12b | 63.37 ± 2.66c | 68.87 ± 9.45c | 111.55 ± 7.24a |
| 2‐Methylbutanal | 395.26 ± 2.48b | 761.38 ± 7.42a | 362.78 ± 6.04c | 359.56 ± 8.58c | 355.68 ± 7.67c | 399.05 ± 26.24d | 800.30 ± 3.72a | 569.86 ± 13.43b | 481.04 ± 14.80c | 185.03 ± 15.50d |
| 3‐Methylbutanal | 466.59 ± 1.29b | 499.93 ± 4.13a | 214.44 ± 6.35c | 168.68 ± 13.57d | 183.05 ± 9.27d | 443.43 ± 8.87b | 520.31 ± 29.67a | 346.11 ± 18.29c | 218.82 ± 18.43d | 91.93 ± 4.49e |
| Alcohols | ||||||||||
| 1‐Hexanol | 248.44 ± 3.18a | 109.37 ± 7.07b | 78.36 ± 7.53c | 88.10 ± 2.52c | 85.23 ± 5.52c | 268.05 ± 5.30a | 99.23 ± 8.09b | 105.23 ± 3.03b | 98.31 ± 1.83b | 72.78 ± 4.52c |
| E‐2‐Hexenol‐M | 259.58 ± 3.61a | 78.81 ± 4.40b | 64.97 ± 3.95c | 53.88 ± 5.48d | 80.76 ± 5.34b | 281.19 ± 4.04a | 63.70 ± 6.22c | 81.93 ± 2.09b | 52.92 ± 4.23d | 48.09 ± 2.45d |
| E‐2‐Hexenol‐D | 2,867.64 ± 36.09a | 216.76 ± 2.47d | 314.55 ± 10.02c | 401.14 ± 12.39b | 392.21 ± 12.87b | 2,604.23 ± 27.02a | 232.49 ± 7.89e | 472.15 ± 28.12b | 382.60 ± 13.48c | 313.76 ± 7.33d |
| 3‐Methylpentanol | 217.96 ± 10.10a | 8.72 ± 2.41b | 7.78 ± 1.00b | 8.30 ± 0.28b | 9.15 ± 0.36b | 239.95 ± 5.68a | 6.57 ± 0.92c | 12.59 ± 2.11b | 8.38 ± 1.95bc | 8.42 ± 0.98bc |
| 1‐Pentanol | 115.87 ± 3.14a | 72.42 ± 3.47b | 45.23 ± 0.77c | 45.69 ± 1.92c | 74.00 ± 5.20b | 108.39 ± 4.93a | 73.84 ± 5.51c | 41.61 ± 3.03e | 53.25 ± 7.89d | 98.81 ± 2.81b |
| 2‐Methylbutanol | 726.89 ± 10.43a | 71.20 ± 0.46c | 94.15 ± 3.98b | 54.59 ± 0.72d | 68.58 ± 1.73c | 769.92 ± 10.83a | 61.32 ± 9.62c | 71.37 ± 7.79c | 61.53 ± 4.59c | 120.63 ± 2.73b |
| 3‐Methylbutanol | 82.91 ± 2.44c | 881.67 ± 75.86b | 1,678.44 ± 33.45a | 1,732.73 ± 60.22a | 1,642.38 ± 51.01a | 83.97 ± 4.37d | 701.33 ± 8.50c | 1,320.41 ± 121.77b | 1,414.22 ± 122.67ab | 1,478.50 ± 36.83a |
| 1‐Butanol | 2,057.89 ± 22.54a | 159.23 ± 13.94c | 213.62 ± 2.98b | 215.31 ± 8.76b | 204.30 ± 5.35b | 2,003.53 ± 35.67a | 163.69 ± 5.56c | 256.78 ± 4.76b | 183.54 ± 12.67c | 157.86 ± 6.32c |
| 2‐Methylpropanol | 74.90 ± 1.80abc | 83.12 ± 6.58a | 64.10 ± 6.23c | 71.23 ± 5.42bc | 81.87 ± 7.78ab | 67.38 ± 3.10b | 80.79 ± 12.07a | 78.07 ± 3.61a | 79.10 ± 3.85a | 54.66 ± 0.20c |
| Ethanol‐M | 773.99 ± 13.26b | 906.03 ± 11.51a | 384.08 ± 13.28c | 370.67 ± 19.75c | 380.81 ± 2.68c | 1,123.28 ± 51.23a | 1,030.45 ± 104.34a | 624.72 ± 33.53b | 542.54 ± 16.16b | 244.02 ± 16.41c |
| Ethanol‐D | 88.59 ± 1.29d | 544.47 ± 13.18c | 1,885.69 ± 70.41a | 1,831.05 ± 43.84a | 1,573.56 ± 40.76c | 136.04 ± 7.10d | 667.01 ± 37.36c | 1,457.70 ± 91.41b | 1,389.34 ± 40.02b | 1,779.19 ± 39.47a |
| Linalool | 307.34 ± 11.22d | 1,540.96 ± 30.63c | 2,312.47 ± 38.35b | 2,315.23 ± 21.20b | 2,423. 01 ± 47.84a | 339.16 ± 17.03c | 1,549.49 ± 8.65b | 2,416.06 ± 23.38a | 2,490.05 ± 8.40a | 2,503.09 ± 105.21a |
| 1‐Octen‐3‐ol | 88.72 ± 1.04b | 126.64 ± 4.72a | 69.07 ± 4.56c | 58.37 ± 5.21d | 71.75 ± 3.36c | 79.98 ± 8.15c | 132.44 ± 4.42a | 107.83 ± 4.79b | 85.97 ± 2.42c | 49.27 ± 9.97d |
| Ketones | ||||||||||
| Acetoin | 1,068.65 ± 16.72d | 1,247.25 ± 3.54c | 1,471.69 ± 23.17b | 1,556.51 ± 13.99a | 1,458.45 ± 17.31b | 1,052.56 ± 2.79d | 1,229.49 ± 31.47c | 1,539.82 ± 10.37a | 1,540.08 ± 26.82a | 1,405.61 ± 37.57b |
| Acetone | 4,182.73 ± 43.16a | 1,782.04 ± 28.14b | 1,291.06 ± 19.61c | 1,098.89 ± 39.74d | 1,036.14 ± 68.25d | 3,835.80 ± 34.11a | 1,740.47 ± 24.98c | 1,977.81 ± 136.24b | 1,539.08 ± 28.29d | 386.61 ± 4.58e |
| Cyclohexanone | 301.37 ± 11.62d | 425.41 ± 3.79a | 377.72 ± 6.84b | 289.24 ± 3.70e | 318.22 ± 1.86c | 306.27 ± 8.33a | 327.62 ± 2.33b | 380.17 ± 5.76a | 281.61 ± 12.24c | 209.76 ± 19.91d |
| 6‐Methyl‐5‐hepten‐2‐one | 237.37 ± 7.95a | 112.55 ± 6.67b | 66.75 ± 4.31c | 66.60 ± 8.27c | 67.71 ± 4.94c | 275.64 ± 10.79a | 89.15 ± 3.22c | 100.01 ± 4.38b | 82.24 ± 2.84c | 53.16 ± 1.72d |
| Furans | ||||||||||
| 2‐Ethylfuran | 151.09 ± 5.03d | 174.04 ± 5.90d | 231.90 ± 8.35c | 443.32 ± 24.77b | 484.44 ± 24.55a | 232.79 ± 3.40d | 160.65 ± 6.18e | 431.02 ± 31.97a | 351.12 ± 32.36b | 299.73 ± 3.15c |
| 2‐Pentylfuran | 83.89 ± 6.23a | 47.01 ± 6.85c | 59.54 ± 1.53b | 50.80 ± 5.36c | 48.02 ± 3.19c | 92.62 ± 9.18a | 57.73 ± 6.06c | 78.26 ± 3.88b | 47.28 ± 4.18cd | 42.22 ± 3.59d |
| 2‐Acetylfuran | 116.14 ± 2.52d | 143.32 ± 7.09c | 139.18 ± 2.80c | 161.21 ± 10.90b | 278.84 ± 14.15a | 109.20 ± 6.03d | 142.58 ± 4.92c | 178.39 ± 3.22b | 143.48 ± 6.29c | 205.64 ± 20.76a |
| Acids | ||||||||||
| 2‐Methylpropionic acid | 980.12 ± 30.62c | 9,593.35 ± 149.27a | 9,592.00 ± 30.64a | 9,576.79 ± 72.2a | 8,732.55 ± 186.26b | 918.27 ± 39.00e | 10,156.44 ± 82.03a | 8,986.20 ± 110.58d | 9,678.86 ± 74.85b | 9,319.22 ± 82.58c |
| 2‐Methylbutanoic acid | 68.92 ± 2.18e | 134.76 ± 3.51d | 525.19 ± 12.09a | 352.67 ± 2.62c | 404.33 ± 8.15b | 70.03 ± 6.08c | 145.99 ± 18.00b | 153.33 ± 22.79b | 197.27 ± 28.50b | 808.58 ± 53.04a |
| Pyrazine | ||||||||||
| 2‐6‐Dimethylpyrazine | 249.48 ± 7.16a | 100.61 ± 2.53b | 84.91 ± 4.95c | 63.36 ± 6.12d | 68.36 ± 3.92d | 237.88 ± 7.17a | 93.85 ± 1.40c | 115.23 ± 5.90b | 61.01 ± 2.88e | 77.49 ± 3.93d |
| Ether | ||||||||||
| Dimethyl disulfide | 1,684.55 ± 14.51a | 286.21 ± 2.08b | 58.01 ± 1.31d | 66.06 ± 1.91d | 90.91 ± 3.98c | 1,623.68 ± 28.53a | 271.58 ± 5.36b | 105.17 ± 9.91c | 88.40 ± 7.71c | 98.90 ± 1.83c |
The significance analysis results were based on the peak volume value of FMP during NF and IF process, and different letters indicated significant differences (p < .05).
Abbreviations: D: dimer; IF: inoculated fermentation; IF‐0, IF‐6, IF‐12, IF‐18, IF‐24: IF samples were obtained on 0, 6, 12, 18, and 24 days; M: monomer; NF: natural fermentation; NF‐0, NF‐6, NF‐12, NF‐18, NF‐24: NF samples were obtained on 0, 6, 12, 18, and 24 days.
Esters, closely related to the fruity and sweet flavor, played an important role in FMP flavor. The change of eight esters during fermentation process is shown in Figure 5a and Table 2. Most of esters decreased with the increasing in fermentation time, including hexyl hexanoate, ethyl nonanoate, methyl octanoate, isoamyl hexanoate, and gamma‐butyrolactone‐M. High‐acid environment might promote the hydrolysis of above esters (Tomita et al., 2018). In the early fermentation, isoamyl hexanoate‐M sharply deceased, but the isoamyl hexanoate‐D sharply increased. This phenomenon indicated that high content of isoamyl hexanoate‐M turned into the isoamyl hexanoate‐D, which was in accordance with the result of fingerprint analysis. In addition, isoamyl hexanoate posed an acid stability and was not easy to hydrolyze at low pH. Hence, isoamyl hexanoate content was the highest in all the eaters during fermentation process. Wang, Wang, Xiao, Liu, Deng, et al. (2019) found that ethyl salicylate, ethyl palmitate, methyl linoleate, ethyl linoleate, and ethyl stearate were the main esters in FMP during fermentation process, but no many isoamyl hexanoate was produced. The reason for the difference in two experiments was in accordance with fermentation conditions, salt content, and pepper varieties. According to these results, it was speculated that isoamyl hexanoate played an important role in the aroma quality of FMP. Ethyl acetate, with pineapple flavor, showed an increased tendency during fermentation process. Microorganisms turned the fermented carbohydrates into the end products during fermentation process, including organic acids and alcohols (Kim et al., 2016). The organic acids and alcohols could combine each other to form several esters, such as ethyl acetate (Wang, Wang, Xiao, Liu, Deng, et al., 2019). It was found that more ethyl acetate was produced during liquor fermentation, and became a key volatile compound of liquor during liquor brewing process (Cai et al., 2019). However, little research reported that ethyl acetate could increase to high level during vegetable fermentation. Hence, ethyl acetate and isoamyl hexanoate were the two main esters at the end of fermentation, and attributed to the FMP flavor.
Aldehydes showed lower threshold values and strong fragrance‐giving ability. For FMP, aldehydes with suitable content were beneficial for the maintenance of pungency flavor. Eight kinds of aldehydes were selected to explore the change in aldehydes during fermentation process, as shown in Figure 5b and Table 2. Most aldehydes decreased with the prolonging of fermentation time, including methional, phenylacetaldehyde, and furfurol, especially for methional and phenylacetaldehyde. During fermentation process, the aldehydes turned into the acids and alcohols under the action of microorganisms, which was an important reason for aldehyde degradation. Meanwhile, condensation could be occurred among aldehydes (Sukharev, Mariychuk, Onysko, Sukhareva, & Delegan‐Kokaiko, 2019), which accelerated aldehyde degradation. The decrease in above aldehydes weakened the pungency of FMP at some extent. Zhao et al. (2015) found that almost all aldehydes, such as butanal, octanal, and others, decreased with the prolonging of pumpkin juice fermentation time. For 2‐methylbutanal, the content increased in the first 6 days, then decreased in the late 18 days. Li, Dong, et al. (2016) explored the relationship between bacterial communities and volatile compounds in red pepper paste, and found that Pseudomonas posed an obvious correlation with 2‐methylbutanal. Hence, it was speculated that the change in 2‐methylbutanal content might be related to Pseudomonas during fermentation process. Hazelwood, Daran, van Maris, Pronk, and Dickinson (2008) found that some aldehydes, such as methylbutanal, could be generated by Strecker degradation of methionine and leucine or the Ehrlich pathway. Although octanal content slightly increased during fermentation process, the content was still lower than most other aldehydes. At the end of fermentation, the content of all aldehydes was low, which weakened the pungency of FMP.
Alcohols, with high threshold values, were mainly generated by alcohol and lactic acid fermentation (Wang, Wang, Xiao, Liu, Deng, et al., 2019). However, the threshold of unsaturated aldehyde was low, which made many contributions to food flavor (Lorenzo, Carballo, & Franco, 2013). As shown in Figure 5c and Table 2, the content of some alcohols increased sharply in the early fermentation time, and then maintained a stable level in the late fermentation time, including linalool, ethanol‐D, and 3‐methylbutanol. It indicated that the ability in early fermentation was stronger than that in late fermentation. In early fermentation time, LAB became the prominent microorganism quickly comparing with other microorganisms, promoted LAB fermentation, and produced some acids and alcohols. Nguyen et al. (2013) also proved that LAB in traditional fermented vegetables of Vietnam could grow rapidly and became the dominant bacteria under suitable conditions during fermentation process. In addition, alcohol fermentation also existed in early fermentation process, and produced some alcohols, especially for ethanol. In late fermentation time, the fermentation ability of microorganism was weakened because of the worst living conditions, such as less nutrient substance (Beganovi et al., 2014). However, some alcohols showed obvious decrease in tendency in early fermentation time, then hardly changed, especially for E‐2‐hexenol‐D and 1‐butanol. E‐2‐hexenol‐D was sensitive to the acids, and easily decomposed or turned into other compounds at low pH. The reason for 1‐butanol degradation might be that 1‐butanol turned into butyric acid at the action of microbial metabolism and oxidation. The increase in 2‐methylbutanoic acid was probably related to above change. At the end of fermentation, linalool content was the highest in all alcohols, and gave flower‐like aroma, which posed an important contribution to the FMP flavor. Linalool was also existed in some fermented vegetable products, such as fermented red pepper pastes and cucumber (Li, Dong, Zhao, & Zhu, 2020; Zhou & McFeeters, 1998). Zhou and McFeeters (1998) found that the content of linalool in fermented cucumber increased to several times than its odor threshold during fermentation.
The other volatile compounds, including 4 ketones, 3 furans, 2 acids, 1 pyrazine, and 1 ether, are shown in Figure 5d,e and Table 2. The content of 2‐methylpropionic acid and 2‐methylbutanoic acid increased after fermentation. In particular for 2‐methylpropionic acid, the content sharply increased to a high level. Whatever NF or IF, lactic acid fermentation was the main fermentation pathway, and involved the oxidation of carbohydrates to other compounds, which caused the production of lots of acids. Li et al. (2020) explored the effect of different salt content (10%, 15%, 20%, and 25%) on microbial categories and related qualities, and found that LAB became the prominent population at high salt content. At the end of fermentation, it was found that the pH value was up to about 4.10, which was similar to our experiment. Ketones possessed a lower threshold value and more contributed to food flavor. Acetone, cyclohexanone, and 6‐methyl‐5‐hepten‐2‐one decreased with the prolonging of fermentation time. In particular for acetone, the peak value decreased from 4,009.27 to 711.38 in the whole fermentation. Sukharev et al. (2019) found that ketone could condensate with aldehydes or other ketones, and condensation reaction might be the main reason for the decrease in ketones during fermentation process. The final peak values of 2‐ethylfuran, 2‐pentyfuran, and 2‐acetylfuran were about 2 times than those of the initial ones. It indicated that some furans were generated during fermentation process. Furans were hardly detected in many fermented foods (Kim et al., 2019). It is indicated that 2‐ethylfuran, 2‐pentyfuran, and 2‐acetylfuran were probably the characteristic volatile compounds in FMP. Only 1 pyrazine and ether were detected. Hence, it was difficult to analyze the change in pyrazine and ether during fermentation process.
As shown in Figure 5a1–d1, it was found that there was similar content in volatile compounds between NF and IF samples. Under suitable fermentation conditions, including temperature, pH, O2, the LAB fermentation was the prominent pathway whatever NF or IF (Sanlier et al., 2017). Many other microorganisms were inhibited by LAB. Hence, two fermentation methods produced the similar volatile compounds, but IF might shorten fermentation period and promote FMP quality. It was proved that the use of inoculated microorganism starters guaranteed the agreeable sensory properties, including volatile compounds (Woutets et al., 2013; Zhao et al., 2015).
In summary, main volatile compounds, including esters, aldehydes, alcohols, and acids, notably changed during fermentation. Almost all esters, aldehydes, and some alcohols posed an obvious decrease, and some alcohols and all acids posed an obvious increase during fermentation process. These results were related to microbial fermentation. However, there were no obvious differences in volatile compounds between NF and IF samples. Hence, further analysis was needed to explore volatile compounds during NF and IF process.
3.5. Analysis based on PCA results
Principal component analysis, a multivariate statistical analysis, was used to turn original correlated variables into linearly uncorrelated variables by several related transformation. These uncorrelated variables obtained by PCA could reflect the relationships among the observed variables (Cirlini et al., 2019; Yao et al., 2015). The correlated variables could be distinguished by the positive or negative score values in PC1 and PC2. Yilmaztekin and Sislioglu (2015) found that most important volatile compounds in fermented and raw European cranberrybush were divided into four uncorrelated parts by PCA, and PCA discriminated the fermentation stage as three groups. In our research, PCA obtained by original date was used to compare the difference in principal compounds (Figure 6). An obvious separation of two principal compounds was found during fermentation process and two fermentation methods (NF and IF). Two principal compounds (PC1 and PC2) were up to 87% in variation of originate date. PC1 was up to 74%, but PC2 was only up to 13% in variation of originate date. As shown in Figure 6, the samples showed a well separation degree each other, and three parts were presented in PCA based on previous analysis and heat map. The higher the separation degree, the lower the correlation in different samples. Samples in different parts posed low correlation. According to PCA, an obvious difference was found comparing samples on 0 days (NF and IF) with other samples (NF and IF), as shown in red frame. PC1 and PC2 of samples on 0 days were the positive score values. The result indicated that fermentation time played a notable influence on volatile compounds of FMP. The other samples could be well distinguished based on the score values of PC1 and PC2. The samples on 6 days were clustered in the yellow frame, but few differences were found between NF and IF samples. Principal compounds of samples on 12, 18, and 24 days of NF and IF were clustered in green frame. The samples on 12 and 18 days during IF have negative score values of PC1 and PC2, but the negative score values of PC1 and the positive score values of PC2 in other samples that were found. According to above results, it was found that there were few differences in principal compounds in the late fermentation time. It is indicated that the late fermentation ability was weakened because of less nutrition substance. In summary, the PCA well discriminated the stage of NF and IF as three groups (0 days; 6 day; and 12, 18, and 24 days). Based on above results, fermentation time played a key role in change of volatile compounds, but the two fermentation methods posed little effect on volatile compounds.
FIGURE 6.
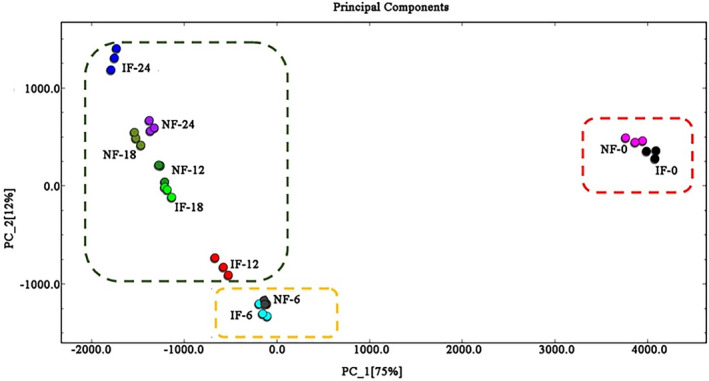
PCA of volatile compounds during NF and IF process. IF: inoculated fermentation; IF‐0, IF‐6, IF‐12, IF‐18, IF‐24: IF samples were obtained on 0, 6, 12, 18, and 24 days; NF: natural fermentation; NF‐0, NF‐6, NF‐12, NF‐18, NF‐24: NF samples were obtained on 0, 6, 12, 18, and 24 days
3.6. Clustering analysis based on heat map
To further analyze the differences in volatile compounds of FMP during fermentation process, the cluster analysis was obtained based on the heat map, as shown in Figure 7. In the heat map, the samples were clustered in the horizon, and volatile compounds were clustered in the vertical. There was the high correlation degree in the same category. The samples showed a higher correlation degree each other with the shortening of Euclidean distance. Based on the vertical mode, all samples were clustered into three main categories. Samples on 0 days were clustered together under NF and IF, as the first category. Hence, NF samples were highly similar with IF ones on 0 days. According to the color depth, samples on 0 days showed high content of volatile compounds, such as hexyl hexanoate, methyl octanoate, gamma‐butyrolactone, phenylacetaldehyde, dimethyl disulfide, and acetone. The second category was the NF and IF samples on 6 day. The other samples presented a high correlation between NF (12–24 days) and IF (12–24 days), as the third category. In the late fermentation time, several volatile compound content was up to a high level, including 2‐methylbutanoic acid, 2‐methylpropionic acid, 2‐acetylfuran, and ethyl acetate. On the basis of vertical analysis in heat map, it could be concluded that volatile compounds changed with the prolonging of fermentation time, especially in the early fermentation time. Above results were similar with PCA and previous analysis. Based on the horizontal mode, all volatile compounds in FMP could be classified into two main categories according to the variation tendency (increase and decrease). In heat map, the content of hexyl hexanoate, methyl octanoate, gamma‐butyrolactone, E‐2‐hexenol, 2‐methylbutanol, dimethyl disulfide, acetone, and 1‐butanol obviously decreased to a low level. Apparently, these volatile compounds were clustered into a category with the shortening of Euclidean distance, as the first category. The result was related to change in microorganisms during fermentation process. However, methyl benzoate, ethyl acetate, linalool, 3‐methylbutanol, acetoin, 2‐ethylfuran, 2‐acetylfuran, 2‐methylpropionic acid, and 2‐methylbutanoic acid were clustered into another category, showing an increase tendency in content. LAB fermentation could turn the carbohydrate to other end products, such as acids and alcohols, so promoted the increase in above volatile compounds. These results from heat map further confirmed that HS‐GC‐IMS was a reliable way to detect the volatile compounds in FMP during NF and IF periods.
FIGURE 7.
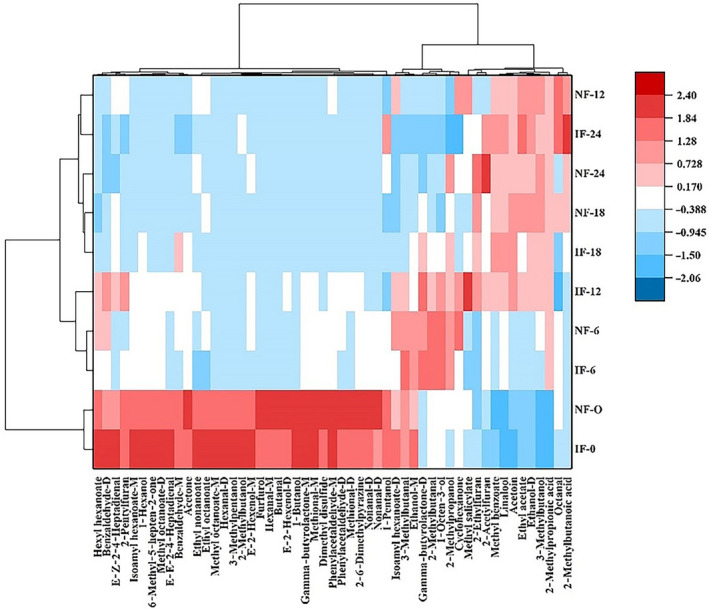
Heat map clustering of volatile compounds during NF and IF process. D: dimer; IF: inoculated fermentation; IF‐0, IF‐6, IF‐12, IF‐18, IF‐24: IF samples were obtained on 0, 6, 12, 18, and 24 days; M: monomer; NF: natural fermentation; NF‐0, NF‐6, NF‐12, NF‐18, NF‐24: NF samples were obtained on 0, 6, 12, 18, and 24 days
4. CONCLUSION
The study investigated the change in volatile compounds of FMP during NF and IF process using the HS‐GC‐IMS instrument and related supplementary analysis software. A total of 53 volatile compounds were identified in all samples, including 12 esters, 17 aldehydes, 13 alcohols, four ketones, three furans, two acids, one pyrazine, and one ether. With the prolonging of fermentation time, most esters, aldehydes, and alcohols obviously decreased, especially for isoamyl hexanoate, methyl octanoate, gamma‐butyrolactone, phenylacetaldehyde, methional, and E‐2‐hexenol. However, 2‐methylbutanoic acid, 2‐methylpropionic acid, linalool, and ethyl acetate increased to a high level during fermentation process. Above volatile compounds were the indicators of flavor at the end of fermentation time, playing a key role in unique flavor of FMP. Hence, the fermentation time possessed an obvious effect on change in volatile compounds. However, volatile compounds in NF and IF samples showed slight differences at the same fermentation time. Based on PCA and heat map, the fermentation process in all samples was well discriminated as three stages (0 days; 6 day; and 12, 18, and 24 days), and all volatile compounds were divided into two categories (increase and decrease). Above results proved that HS‐GC‐IMS was an effective method to analyze the change in volatile compounds in FMP during NF and IF process.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
HUMAN AND ANIMAL RIGHTS
No animals or humans were used for studies that are the basis of this manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 31601525), Natural Science Foundation of Hunan Province (2019JJ50256), the Introduces Talent Projects of Hunan Agricultural University (20654/540490316002), and Funds for Distinguished Young Scientists of Changsha (KQ1905025).
Chen Y, Xu H, Ding S, et al. Changes in volatile compounds of fermented minced pepper during natural and inoculated fermentation process based on headspace–gas chromatography–ion mobility spectrometry. Food Sci Nutr. 2020;8:3362–3379. 10.1002/fsn3.1616
Yuyu Chen and Haishan Xu contributed equally to this work.
REFERENCES
- Arce, L. , Gallegos, J. , Garrido, R. , Medina, L. M. , Sielemann, S. , & Wortelmann, T. (2014). Ion mobility spectrometry a versatile analytical tool for metabolomics applications in food science. Current Metabolomics, 2(4), 264–271. 10.2174/2213235X03999150212102944 [DOI] [Google Scholar]
- Arroyo‐Manzanaresa, N. , Martín‐Gómeza, A. , Jurado‐Camposa, N. , Garrido‐Delgadoa, R. , Arceb, C. , & Arce, L. (2018). Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace‐gas chromatography‐ion mobility spectrometry. Food Chemistry, 246, 65–73. 10.1016/j.foodchem.2017.11.008 [DOI] [PubMed] [Google Scholar]
- Bautista‐Expósito, S. , Peñas, E. , Silván, J. M. , Frias, J. , & Martínez‐Villaluenga, C. (2018). pH‐controlled fermentation in mild alkaline conditions enhances bioactive compounds and functional features of lentil to ameliorate metabolic disturbances. Food Chemistry, 248, 262–271. 10.1016/j.foodchem.2017.12.059 [DOI] [PubMed] [Google Scholar]
- Beganovi, J. , Kos, B. , Leboš Pavunc, A. , Uroic, K. , Jokic, M. , & Šuškovic, J. (2014). Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiological Research, 169(7–8), 623–632. 10.1016/j.micres.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Cai, X. M. , Shen, Y. , Chen, M. Y. , Zhong, M. Y. , Zhou, Y. L. , & Luo, A. M. (2019). Characterisation of volatile compounds in Maotai flavour liquor during fermentation and distillation. Journal of the Institute of Brewing, 125(4), 453–463. 10.1002/jib.581 [DOI] [Google Scholar]
- Cirlini, M. , Luzzini, G. , Morini, E. , Folloni, S. , Ranieri, R. , Dall'Asta, C. , & Galaverna, G. (2019). Evaluation of the volatile fraction, pungency and extractable color of different Italian Capsicum annuum cultivars designed for food industry. European Food Research and Technology, 245, 2669–2678. 10.1007/s00217-019-03378-x [DOI] [Google Scholar]
- Esteban‐Torres, M. , Landete, J. M. , Reverón, I. , Santamaría, L. , de las Rivas, B. , & Muñoz, R. (2015). A Lactobacillus plantarum esterase active on a broad range of phenolic esters. Applied and Environmental Microbiology, 81(9), 3235–3242. 10.1128/aem.00323-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . (2018). Available from: http://www.fao.org/faostat/en/#data/QC [last accessed April 6, 2020].
- Ge, S. , Chen, Y. Y. , Ding, S. H. , Zhou, H. , Jiang, L. W. , Yi, Y. J. , … Wang, R. R. (2020). Changes in volatile flavor compounds of peppers during hot air drying process based on headspace‐gas chromatography‐ion mobility spectrometry (HS‐GC‐IMS). Journal of the Science of Food and Agriculture, 100(7), 3087–3098. 10.1002/jsfa.10341 [DOI] [PubMed] [Google Scholar]
- Gobbetti, M. , Di Cagno, R. , & De Angelis, M. (2010). Functional microorganisms for functional food quality. Critical Reviews in Food Science and Nutrition, 50(8), 716–727. 10.1080/10408398.2010.499770 [DOI] [PubMed] [Google Scholar]
- Hazelwood, L. A. , Daran, J. M. , van Maris, A. J. A. , Pronk, J. T. , & Dickinson, J. R. (2008). The ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Applied and Environmental Microbiology, 74(12), 2259–2266. 10.1128/aem.02625-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Carrión, M. , Sanz, T. , Hernando, I. , Llorca, E. , Fiszman, S. M. , & Quiles, A. (2015). New formulations of functional white sauces enriched with red sweet pepper: A rheological, microstructural and sensory study. European Food Research and Technology, 240(6), 1187–1202. 10.1007/s00217-015-2422-1 [DOI] [Google Scholar]
- Hu, X. , Wang, R. R. , Guo, J. J. , Ge, K. D. , Li, G. Y. , Fu, F. H. , … Shan, Y. (2019). Changes in the volatile components of candied kumquats in different processing methodologies with headspace‐gas chromatography‐ion mobility spectrometry. Molecules, 24(17), 1–19. 10.3390/molecules24173053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, K. M. , & Baek, H. H. (2014). Aroma quality assessment of Korean fermented red pepper paste (gochujang) by aroma extract dilution analysis and headspace solid‐phase microextraction‐gas chromatography‐olfactometry. Food Chemistry, 145, 488–495. 10.1016/j.foodchem.2013.08.087 [DOI] [PubMed] [Google Scholar]
- Kim, B. , Hong, V. M. , Yang, J. , Hyun, H. , Im, J. J. , Hwang, J. , … Kim, J. E. (2016). A review of fermented foods with beneficial effects on brain and cognitive function. Preventive Nutrition and Food Science, 21(4), 297–309. 10.3746/pnf.2016.21.4.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Lee, S. , Bang, E. , Lee, S. , Rhee, J. , & Na, Y. (2019). Comparative evaluation of flavor compounds in fermented green and roasted coffee beans by solid phase microextraction‐gas chromatography/mass spectrometry. Flavour and Fragrance Journal, 34(5), 365–376. 10.1002/ffj.3517 [DOI] [Google Scholar]
- Lantsuzskaya, E. V. , Krisilov, A. V. , & Levina, A. M. (2015). Structure of aldehyde cluster ions in the gas phase, according to data from ion mobility spectrometry and ab initio calculations. Russian Journal of Physical Chemistry A, 89(9), 1590–1594. 10.1134/s0036024415090204 [DOI] [Google Scholar]
- Li, M. Q. , Yang, R. W. , Zhang, H. , Wang, S. L. , Chen, D. , & Lin, S. Y. (2010). Development of a flavor fingerprint by HS‐GC‐IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chemistry, 290, 32–39. 10.1016/j.foodchem.2019.03.124 [DOI] [PubMed] [Google Scholar]
- Li, Y. J. , Zhao, F. , Liu, H. H. , Li, R. J. , Wang, Y. T. , & Liao, X. J. (2016). Fermented minced pepper by high pressure processing, high pressure processing with mild temperature and thermal pasteurization. Innovative Food Science and Emerging Technologies, 36, 34–41. 10.1016/j.ifset.2016.05.012 [DOI] [Google Scholar]
- Li, Z. , Dong, L. , Huang, Q. , & Wang, X. (2016). Bacterial communities and volatile compounds in Doubanjiang, a Chinese traditional red pepper paste. Journal of Applied Microbiology, 120(6), 1585–1594. 10.1111/jam.13130 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Dong, L. , Jeon, J. , Kwon, S. Y. , Zhao, C. , & Baek, H. H. (2019). Characterization and evaluation of aroma quality in doubanjiang, a Chinese traditional fermented red pepper paste, using aroma extract dilution analysis and a sensory profile. Molecules, 24(17), 1–12. 10.3390/molecules24173107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. H. , Dong, L. , Zhao, C. , & Zhu, Y. Q. (2020). Metagenomic insights into the changes in microbial community and antimicrobial resistance genes associated with different salt content of red pepper (Capsicum annuum L.) sauce. Food Microbiology, 85, 1–10. 10.1016/j.fm.2019.103295 [DOI] [PubMed] [Google Scholar]
- Liu, L. , She, X. , Qian, Y. , Li, Y. L. , Tao, Y. F. , Che, Z. M. , … Rao, Y. (2019). Effect of different fermenting containers on the deterioration of Sichuan pickle. LWT‐Food Science and Technology, 111, 829–836. 10.1016/j.lwt.2019.05.024 [DOI] [Google Scholar]
- Lorenzo, J. M. , Carballo, J. , & Franco, D. (2013). Effect of the inclusion of chestnut in the finishing diet on volatile compounds of dry‐cured ham from Celta pig breed. Journal of Integrative Agriculture, 12(11), 2002–2012. 10.1016/s2095-3119(13)60638-3 [DOI] [Google Scholar]
- Nguyen, D. T. L. , Van Hoorde, K. , Cnockaert, M. , De Brandt, E. , Aerts, M. , Binh Thanh, L. , & Vandamme, P. (2013). A description of the lactic acid bacteria microbiota associated with the production of traditional fermented vegetables in Vietnam. International Journal of Food Microbiology, 163(1), 19–27. 10.1016/j.ijfoodmicro.2013.01.024 [DOI] [PubMed] [Google Scholar]
- Ribes‐Moya, A. M. , Raigón, M. D. , Moreno‐Peris, E. , Fita, A. , & Rodríguez‐Burruezo, A. (2018). Response to organic cultivation of heirloom Capsicum peppers: Variation in the level of bioactive compounds and effect of ripening. PLoS ONE, 13(11), 1–24. 10.1371/journal.pone.0207888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlier, N. , Gökcen, B. B. , & Sezgin, A. C. (2017). Health benefits of fermented foods. Critical Reviews in Food Science and Nutrition, 59(3), 506–527. 10.1080/10408398.2017.1383355 [DOI] [PubMed] [Google Scholar]
- Sukharev, S. , Mariychuk, R. , Onysko, M. , Sukhareva, O. , & Delegan‐Kokaiko, S. (2019). Fast determination of total aldehydes in rainwaters in the presence of interfering compounds. Environmental Chemistry Letters, 17, 1405–1411. 10.1007/s10311-019-00875-z [DOI] [Google Scholar]
- Sun, X. , Gu, D. Y. , Fu, Q. B. , Gao, L. , Shi, C. H. , Zhang, R. T. , & Qiao, X. G. (2019). Content variations in compositions and volatile component in jujube fruits during the blacking process. Food Science and Nutrition, 7, 1387–1395. 10.1002/fsn3.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita, S. , Nakamura, T. , & Okada, S. (2018). NMR‐ and GC/MS‐based metabolomic characterization of sunki, an unsalted fermented pickle of turnip leaves. Food Chemistry, 258, 25–34. 10.1016/j.foodchem.2018.03.038 [DOI] [PubMed] [Google Scholar]
- Wang, J. J. , Wang, R. R. , Xiao, Q. , Liu, C. G. , Deng, F. M. , & Zhou, H. (2019). SPME/GC‐MS characterization of volatile compounds of Chinese traditional‐chopped pepper during fermentation. International Journal of Food Properties, 22(1), 1863–1872. 10.1080/10942912.2019.1684320 [DOI] [Google Scholar]
- Wang, J. J. , Wang, R. R. , Xiao, Q. , Liu, C. G. , Jiang, L. , Deng, F. M. , & Zhou, H. (2019). Analysis of bacterial diversity during fermentation of Chinese traditional fermented chopped pepper. Letter in Applied Microbiology, 69(5), 346–352. 10.1111/lam.13212 [DOI] [PubMed] [Google Scholar]
- Woutets, D. , Bernaert, N. , Anno, N. , Van Droogenbroeck, B. , De Loose, M. , Van Bockstaele, E. , & De Vuyst, L. (2013). Application' and validation of autochthonous lactic acid bacteria starter cultures for controlled leek fermentations and their influence on the antioxidant properties of leek. International Journal of Food Microbiology, 165(2), 121–133. 10.1016/j.ijfoodmicro.2013.04.016 [DOI] [PubMed] [Google Scholar]
- Wu, R. W. , Yu, M. L. , Liu, X. Y. , Meng, L. S. , Wang, Q. Q. , Xue, Y. T. , … Yue, X. Q. (2015). Changes in flavour and microbial diversity during natural fermentation of suan‐cai, a traditional food made in Northeast China. International Journal of Food Microbiology, 211, 23–31. 10.1016/j.ijfoodmicro.2015.06.028 [DOI] [PubMed] [Google Scholar]
- Yang, L. Z. , Liu, J. , Wang, X. Y. , Wang, R. R. , Ren, F. , Zhang, Q. , … Ding, S. H. (2019). Characterization of volatile component changes in jujube fruits during cold storage by using headspace‐gas chromatography‐ion mobility spectrometry. Molecules, 24(21), 1–21. 10.3390/molecules24213904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, Y. Z. , Pan, S. , Fan, G. , Dong, L. , Ren, J. , & Zhu, Y. (2015). Evaluation of volatile profile of Sichuan dongcai, a traditional salted vegetable, by SPME‐GC‐MC and E‐nose. LWT‐Food Science and Technology, 64(2), 528–535. 10.1016/j.lwt.2015.06.063 [DOI] [Google Scholar]
- Yilmaztekin, M. , & Sislioglu, K. (2015). Changes in volatile compounds and some physicochemical properties of European cranberrybush (Viburnum opulus L.) during ripening through traditional fermentation. Journal of Food Science, 80(4), 687–694. 10.1111/1750-3841.12836 [DOI] [PubMed] [Google Scholar]
- Yu, Z. H. , Zhang, X. , Li, S. Y. , Li, C. Y. , Li, D. , & Yang, Z. N. (2013). Evaluation of probiotic properties of Lactobacillus plantarum strains isolated from Chinese sauerkraut. World Journal of Microbiology and Biotechnology, 29, 489–498. 10.1007/s11274-012-1202-3 [DOI] [PubMed] [Google Scholar]
- Zhang, L. X. , Shuai, Q. , Li, P. W. , Zhang, Q. , Ma, F. , Zhang, W. , & Ding, X. X. (2016). Ion mobility spectrometry fingerprints: A rapid detection technology for adulteration of sesame oil. Food Chemistry, 192, 60–66. 10.1016/j.foodchem.2015.06.096 [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Liu, W. , Chen, D. , Zhou, C. L. , Song, Y. , Zhang, Y. Y. , … Li, Q. H. (2015). Microbiological and physicochemical analysis of pumpkin juice fermentation by the basidiomycetous fungus Ganoderma lucidum . Journal of Food Science, 80(2), 241–251. 10.1111/1750-3841.12741 [DOI] [PubMed] [Google Scholar]
- Zhou, A. , & McFeeters, R. F. (1998). Volatile compounds in cucumbers fermented in low‐salt conditions. Journal of Agricultural and Food Chemistry, 46(6), 2117–2122. 10.1021/jf9704726 [DOI] [Google Scholar]


