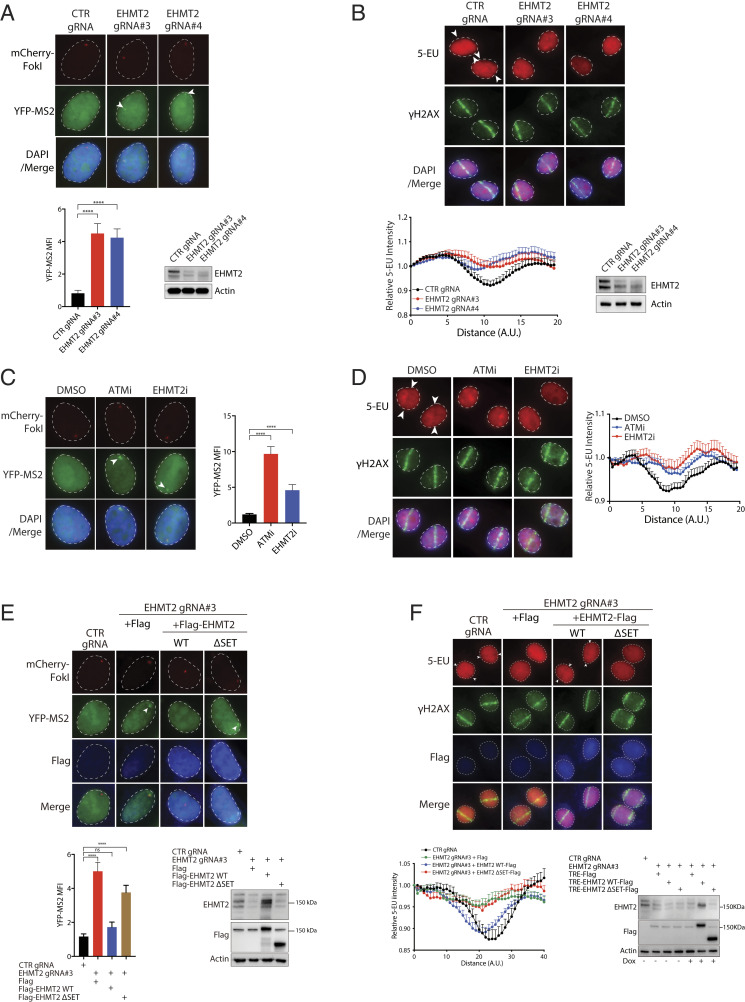
Fig. 6.
EHMT2 promotes transcription silencing on damaged chromatin. (A) EHMT2 inactivation compromised DSB-induced transcription silencing. U2OS-DSB reporter cells lentivirally transduced with the indicated gRNAs were incubated with Dox, 4-OHT, and Shield-1 to induce DSBs proximal to the transcription unit. Thereafter, cells were processed to visualize YFP-MS2 and mCherry-FokI foci. Nuclei were counterstained with DAPI. Arrowheads denote YFP-MS2 foci. MFI of YFP-MS2 was quantified. Bars represent mean ± SEM; ****P < 0.0001. Western blotting was performed to evaluate expression of EHMT2. (B) EHMT2 silencing led to sustained nascent transcription at laser-induced DSBs. HeLa cells transduced with the indicated gRNAs were laser microirradiated. Cells were processed 1 h after to evaluate 5-EU incorporation at laser-induced DNA damage tracks. Arrowheads denote sites of laser microirradiation. Quantification of 5-EU incorporation at laser-induced γH2AX-marked DSBs was performed. Data represent mean ± SEM from three independent experiments. Western blotting was performed to evaluate expression of EHMT2. (C and D) Chemical inhibition of EHMT2 attenuated DISC. Cells pretreated with either ATM inhibitor (KU55933) or EHMT2 inhibitor (UNC0638) were processed for visualization of mCherry-FokI and YFP-MS2 (C) or 5-EU incorporation assay (D). Representative images are shown. Arrowheads denote YFP-MS2 focus or anti–5-EU stripes. Data represent mean ± SEM from three independent experiments. (E and F) Genetic inactivation of EHMT2 methyltransferase activity compromised transcription silencing proximal to FokI- (E) and laser-induced (F) DSBs. Control gRNA- or EHMT2 gRNA-targeted cells reconstituted with vector control, WT EHMT2, or its SET deletion mutant (ΔSET) were subjected to either the U2OS-DSB reporter assay as in A or the 5-EU incorporation assay as in B. Quantification and Western blotting analyses were performed as in A and B. Note that the EHMT2 antibodies were raised against a synthetic peptide corresponding to the carboxyl terminus of the protein, and do not recognize the EHMT2 SET deletion mutant.