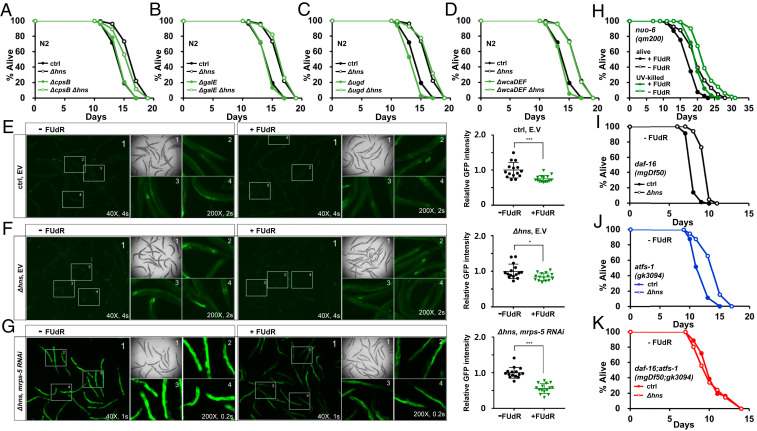
Fig. 3.
Δhns E. coli mutants activate both DAF-16 and UPRmt in C. elegans. (A–D) Mutations leading to deficiencies in CA synthesis did not fully suppress the lifespan extension by Δhns E. coli mutants. The results shown are from one of three independent experiments. (E–G) FUdR reduced the GFP intensity induced by UPRmt. The expression of the hsp-6p::gfp, UPRmt reporter was compared with that of the non–FUdR-treated control. Δhns E. coli synergistically enhanced hsp-6p::gfp expression with mrps-5 RNAi, a mild UPRmt inducer (G). GFP intensity was quantified using ImageJ. *P < 0.05; ***P < 0.001, Student’s t test. Ctrl and Δhns represent HT115 and Δhns HT115 E. coli, respectively. One of three independent experiments is shown. (H) FUdR reduced the lifespan of long-lived nuo-6 (qm200) mutants. This suggests that FUdR acts directly on the mitochondrial longevity pathway in C. elegans, rather than affecting E. coli physiology. One of two independent experiments is shown. (I–K) Lifespan assays of daf-16(mgDf50) (I), atfs-1(gk3094) (J), and daf-16(mgDf50); atfs-1(gk3094) mutants (K) were performed in the absence of FUdR. Neither daf-16(mgDf50) nor atfs-1(gk3094) mutants suppressed the lifespan extension by Δhns E. coli mutants, while the daf-16(mgDf50); atfs-1(gk3094) double mutation fully suppressed lifespan extension. One of at least two independent experiments is shown.