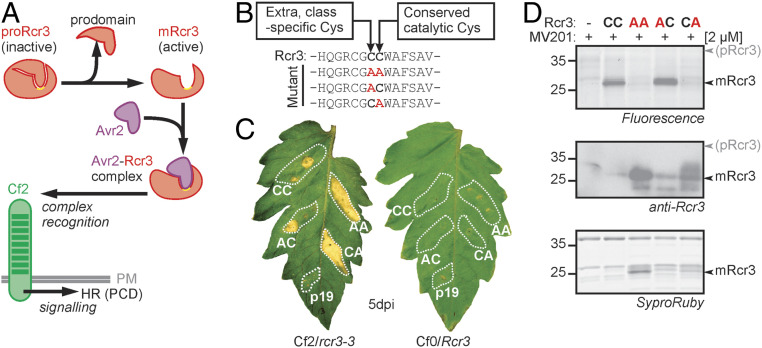
Fig. 1.
Catalytic Rcr3 mutants trigger HR and are processed into mature Rcr3 (mRcr3). (A) Proposed mechanism of Avr2 perception by Rcr3 and Cf-2. The prodomain of Rcr3 is removed to create a binding site for Avr2 and to form a complex that is recognized by the receptor-like protein Cf-2 in the plasma membrane (PM), resulting in HR, a form of programmed cell death. (B) Substitutions of two Cys residues in the active site Rcr3. Rcr3 carries a class-specific Cys residue directly preceding the catalytic Cys residue (CC; bold). All three combinations of Cys-to-Ala substitutions were generated. All Rcr3 constructs were driven by a 35S promoter and cloned into the transfer DNA of a binary plasmid. (C) Catalytic Rcr3 mutants can still trigger HR. AF isolated from agroinfiltrated N. benthamiana plants expressing (mutant) Rcr3 or p19 was coinjected with 300 nM purified Avr2 into leaflets of MM-Cf2/rcr3-3 and MM-Cf0/Rcr3 tomato plants, and pictures were taken at 5 d postinjection (dpi). (D) Catalytic Rcr3 mutants are still processed. AF isolated from agroinfiltrated N. benthamiana plants expressing (mutant) Rcr3 was labeled with 2 µM MV201 for 3.5 h. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by fluorescence scanning (Top), Western blot with an anti-Rcr3 antibody (Middle), and Sypro Ruby staining (Bottom). Rcr3 mutants lacking the catalytic Cys are inactive but accumulate as mature proteins.