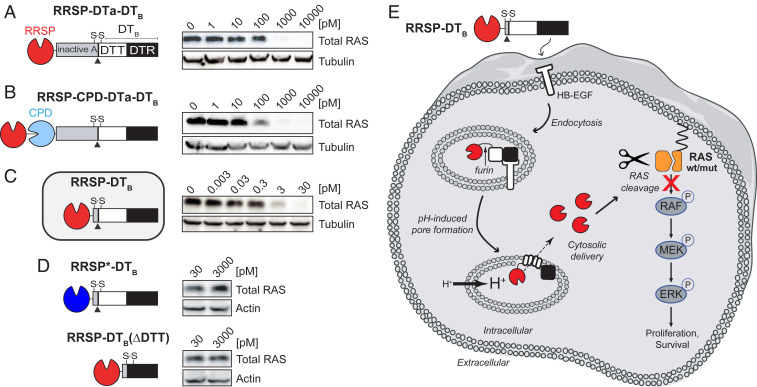
Fig. 1.
Engineered diphtheria toxin delivery machinery enables intracellular delivery of RRSP. Schematic of chimeric fusions of RRSP to (A) the amino terminus of nontoxic, full-length diphtheria toxin (RRSP-DTa-DTT-DTR), (B) to autoprocessing cysteine protease domain (CPD) of MARTX toxin from V. vulnificus (RRSP-CPD-DTa-DTT-DTR), and (C) to DTT-DTR without the DTa domain (RRSP-DTT-DTR or RRSP-DTB) and corresponding immunoblots against total RAS of lysates prepared from HCT-116 cells treated with RRSP-DT variants for 24 h. (D) Immunoblots showing that the catalytically dead RRSP*-DTB mutant and the translocation-deficient control (RRSP-DTB[ΔDTT]) do not affect RAS levels in HCT-116 cells. (E) Schematic diagram illustrating transport and intracellular trafficking of RRSP fused to the translocation T and receptor R binding domains of diphtheria toxin fragment B (DTB) via a receptor-mediated endocytic mechanism. Once in the cytosol, RRSP cleaves RAS resulting in down-regulation of the MAPK/ERK-signaling cascade. Empty cell and vesicles images credit: Servier Medical Art (https://smart.servier.com), which is licensed under CC BY 3.0.