Figure 4. The hCASP1-mGSDMD interface plays an important role in their association.
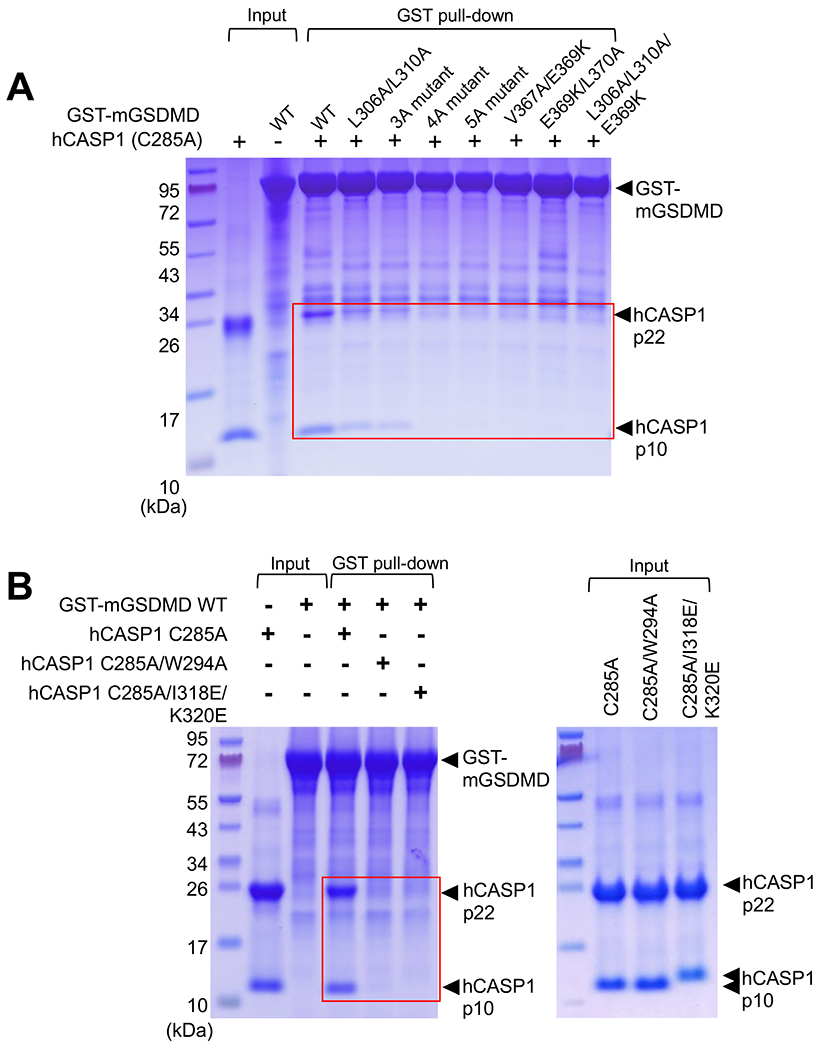
(A) Wild type or mutant GST-mGSDMD was used to pull down the hCASP1 (C285A) p22/p10 catalytic domain. The mutants harbor mutations of the exosite-binding residues in mGSDMD. Data shown are representative of three independent experiments.
(B) Wild type GST-mGSDMD was used to pull down hCASP1 catalytic domains harboring mutations such as C285A, W294A, I318E or K320E. Data shown are representative of three independent experiments.
See also Figure S4.
