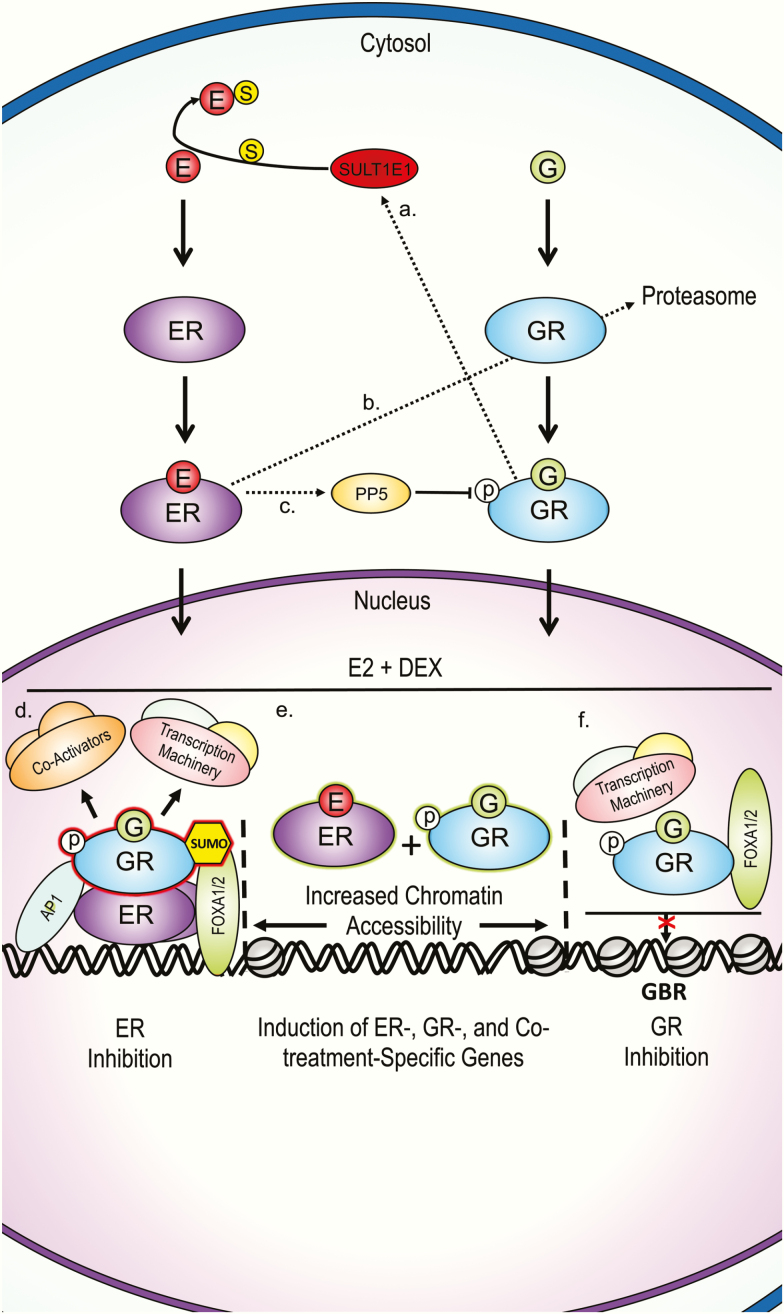
Figure 1.
Summary of proposed G and estrogen cellular crosstalk mechanisms from multiple tissues. A, Gs upregulate SULTE1, which reduces estrogen bioavailability by sulfation in human and mouse hepatocytes, and human breast cancer MCF-7 cells [43]. B, Estrogen promotes GR proteasomal degradation by upregulating p53 and the E3 ubiquitin ligase Mdm2 in MCF-7 cells [44]. C, Estrogen upregulates PP5, which reduces G action by dephosphorylating GR Ser-211 in breast cancer cell lines [45]. D, GR inhibits ER transcription assembly for some genes. DNA-bound ER is bound by GRs DBD. AP-1 and FOXA1 assist in GR-ER association in breast cancer cell lines [47, 49]. GR SUMOylation is needed for destabilization of transcription complexes in certain enhancers [49]. E, Co-treatment results in increased chromatin accessibility and unique ER and GR genome binding and consequent gene expression not observed during either single hormone treatment in breast and uterine cancer cell lines [50, 51, 62]. F, Estrogen inhibits GR target gene expression for some genes, characterized by lower chromatin accessibility and consequent lower GR, FOXA1/2, and transcription machinery binding to GBRs in human uterine cells [59, 60]. DBD, DNA-binding domain; DEX, dexamethasone; E, estrogen; ER, estrogen receptor; G, glucocorticoid; GBR, glucocorticoid-binding region, GR, glucocorticoid receptor; p, phosphate; PP5, protein phosphatase 5; SULT1E1, estrogen sulfotransferase; S, sulfate.