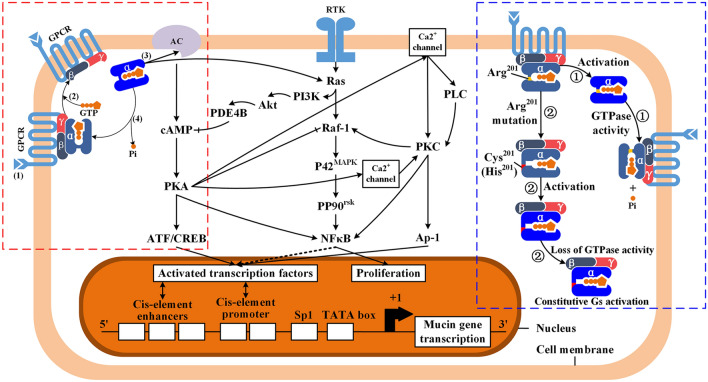
Fig. 3.
G protein cycle, activity changes of Gsα subunit caused by GNAS mutation, and the crosslink among Gsα subunit-induced cAMP–PKA, PI3K–Akt, and RAS–MAPK pathways. Red dotted box: G protein cycle; blue dotted box: activity changes of Gsα subunit caused by the mutation of Gsα Arg201. ① In the physiological status, activated Gsα returns to deactivated status after releasing a Pi; ② In the situation of Arg201 mutation, Gsα fails to release Pi and remains in activated status. Gsα in ink blue: deactivated status. Gsα in light blue: activated status; the other signaling pathways: cAMP–PKA, PI3K–Akt, and RAS–MAPK pathways interacts among each other and eventually modulate mucin gene expression via the nuclear import of ATF/CREB and NFκB. GPCR G protein-coupled receptor, Gsα stimulatory G protein subunit, Pi inorganic phosphate, AC adenyl cyclase, cAMP cyclic adenosine monophosphate, PKA protein kinase A, ATF activating transcription factor, CREB cAMP-response element-binding protein, PLC phospholipase C, PKC protein kinase C, PI3K phosphoinositide 3-kinase, Akt protein kinase B, PDE4B phosphodiesterase 4B, RTK receptor tyrosine kinases, Ras rat sarcoma protein, Raf-1 Raf-1 protein, P42MAPK P42 mitogen-activated protein kinas, also named Erk2, extracellular signal-regulated kinase 2, PP90rsk 90 kDa ribosomal S6 kinase, NFκB nuclear factor kappa-light-chain-enhancer of activated B cells, Sp1 specificity protein 1, Arg arginine, Cys cysteine, His histidine