Figure 3.
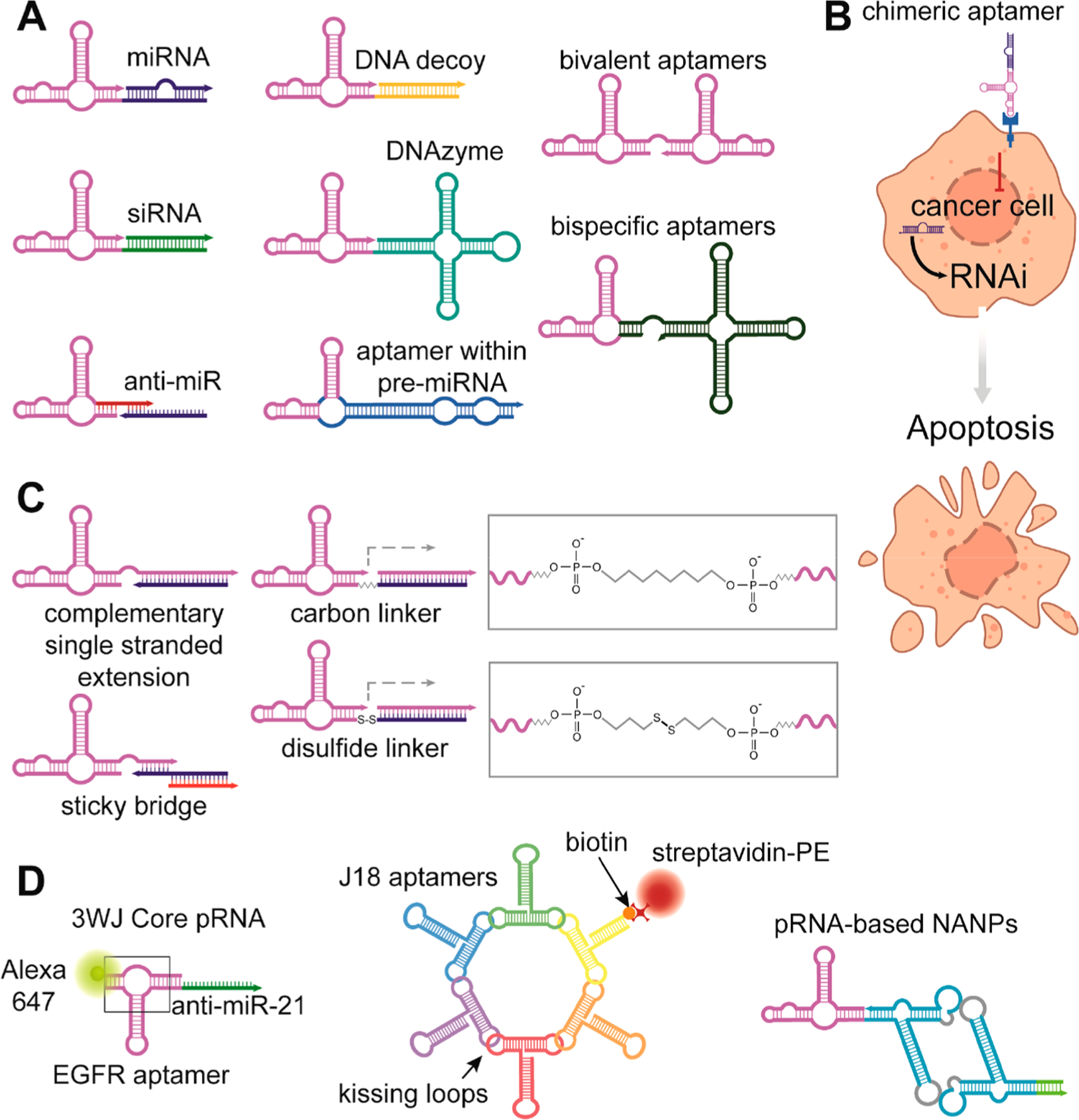
Schematic depiction of various chimeric aptamers. Multiple nucleic-acid-based functionalities can be linked to cell-specific aptamers with many different approaches that are beyond the scope of the article. (A) Post-transcriptional silencing of gene expression is achieved by delivery of miRNA or siRNA. Transcription of aptamers within pre-miRNA from gene constructs offers prolonged production of chimeric RNA. Downregulation of some genes by endogenous miRNAs during tumorigenesis can be reverted by the delivery of anti-miRs that block binding of miRNAs to target mRNAs. Delivery of a DNAzyme that cleaves specific mRNA is another way to repress gene expression. Conjugation of two identical aptamers is used for oligomerization of receptors in comparison with two different aptamers that may attach to cell surface proteins from intercellular space or the bloodstream as well as interconnect two different cells. (B) Schematic illustration of cell targeting by chimeric aptamer with a synergistic effect. An aptamer blocks signaling, while the therapeutic payload silences genes crucial for cell survival. Thus, simultaneously both functional parts promote apoptosis. (C) Most utilized ways of chimeric TNA conjugations. (D) From left to right: 3WJ-EGFR aptamer/anti-miR-21 nanoparticles harboring three functional modules: EGFR RNA aptamer for targeted delivery, anti-miR-21 LNA for therapy, and Alexa-647 dye for imaging. The RNA nanoring carrying five J18 aptamers for cell targeting, connected to the RNA ring. One biotinylated oligonucleotide provides fluorescent readout after coupling to a streptavidin–phycoerythrin conjugate. The 3WJ pRNA motif can be used to multiply assemble scaffolds.
