Figure 2.
JAM-A Regulates Actomyosin Remodeling
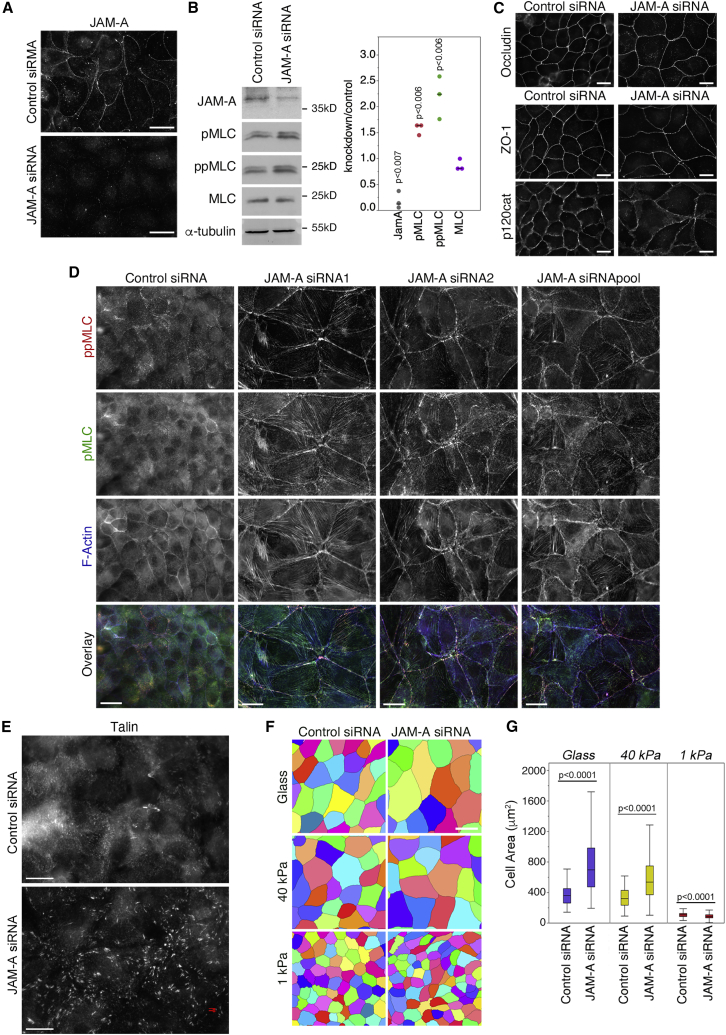
(A and B) Depletion of JAM-A in MDCK cells was induced by transfection of siRNAs and was monitored by immunofluorescence (A) or immunoblotting (B).
(C) Control and JAM-A-depleted cells were stained for markers of tight (occludin and ZO-1) and adherens junctions (p120-catenin).
(D and E) MDCK cells were transfected with either control or JAM-A-targeting siRNAs before fixation and staining for double- and single-phosphorylated MLC to reveal active NMMII and F-actin (D) or talin to reveal focal adhesions (E).
(F and G) Cells transfected with siRNAs were plated on Matrigel-coated coverslips or 40 kPa or 1 kPa hydrogels before immunofluorescence. The apical surface area was then quantified as a measure for cell spreading by obtaining a cell segmentation based on ZO-1 staining (glass control siRNA, 81 cells; JAM-A siRNA, 55 cells; 40 kPa control siRNA, 102 cells; and JAM-A, 73 cells; 1 kPa control siRNA, 298 cells; and JAM-A, 287 cells; box-plot shows median and the interquartile range). Magnification bars, 20 μm. See also Figure S2.