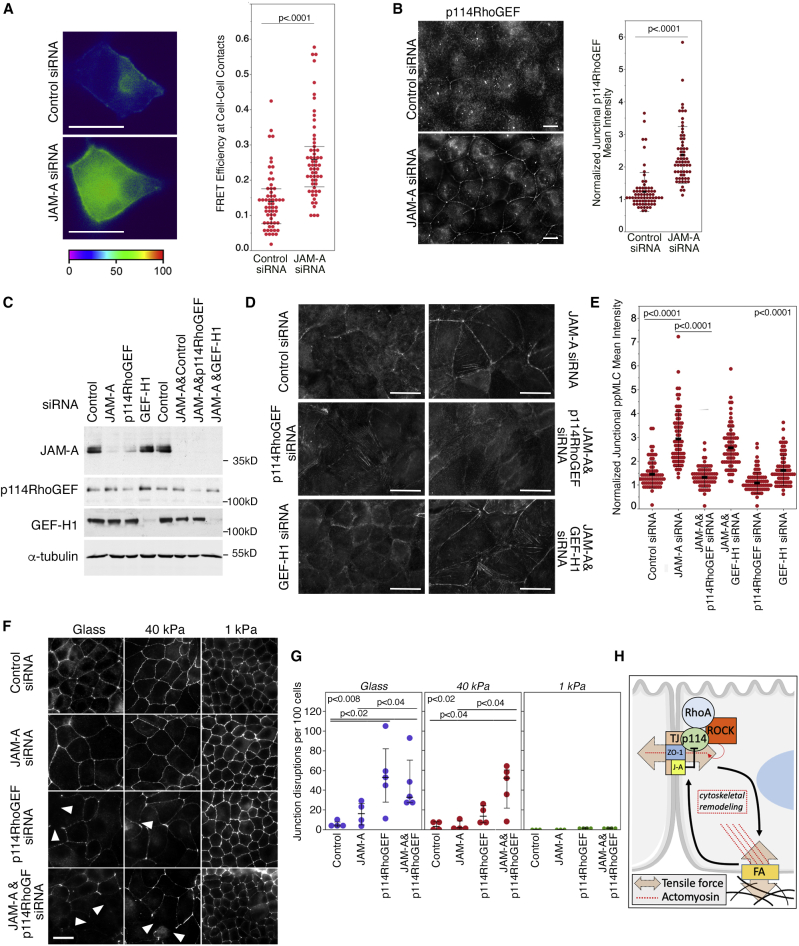
Figure 4.
p114RhoGEF Regulates Junctional Actomyosin Remodeling and Tight Junction Assembly in JAM-A-Depleted Cells
(A) Control and JAM-A-depleted cells were transfected with a FRET biosensor for RhoA activation, and FRET efficiency at cell-cell contacts was quantified (n for both conditions was 58; shown are the data values along with the median and the interquartile range).
(B) Control and JAM-A-depleted cells were stained for p114RhoGEF, and junctional recruitment was quantified (n for control siRNA, 68; JAM-A siRNA, 63; shown are datapoints and means ± 1 SD).
(C–E) JAM-A and the Rho GEFs p114RhoGEF and GEF-H1 were depleted individually or combined as indicated prior to immunoblotting (C) or immunofluorescence (D). (D) Shows staining for double-phosphorylated MLC, a quantification of which is shown in (E) (n = 73 for all categories; shown are datapoints and means ± 1 SD). See Figure S3B for images of F-actin and single-phosphorylated MLC.
(F and G) Tight junction formation was assessed by quantifying occludin staining disruptions in siRNA-transfected cells plated on Matrigel-coated glass coverslips or 40 kPa or 1 kPa hydrogels (F). Examples of disrupted tight junctions are labeled by arrowheads. Numbers of junctional disruptions per 100 cells were manually counted (datapoints correspond to single fields analyzed; also shown are the median and the interquartile range; >100 cells were analyzed for each category) (G).
(H) Scheme summarizing the mechanism of regulation of tensile stress on tight junctions by JAM-A and ECM. Magnification bars, 20 μm. See also Figures S3 and S4.