Figure 1.
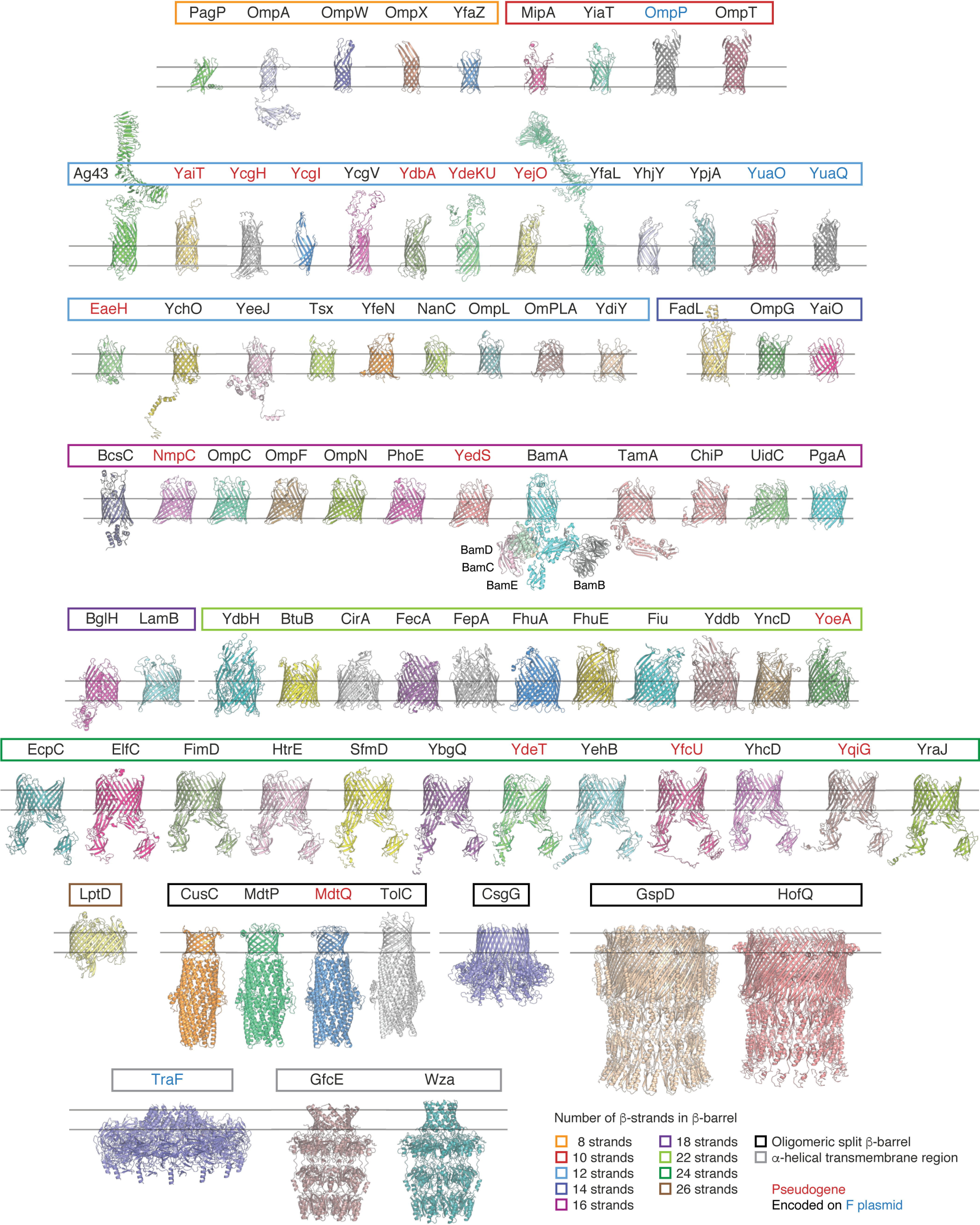
Structures of transmembrane proteins found in the OM of E. coli K-12 MG1655. A list of all known and predicted transmembrane proteins in the OM of E. coli K-12 strain MG1655 was manually curated, creating the “OMP-ome.” The Protein Data Bank was then searched for solved structures of these proteins or close homologues. Where no high-resolution solved 3D structures were available, homology models were generated using the I-TASSER server (RRID:SCR_014627) (396). For two proteins, NfrA (the N4 bacteriophage receptor), and FlgH (the flagellar L-ring protein), no homology models could be generated. Predictions for YaiO, YcgI, YdbH, and YhjY generated deformed or broken barrels (possibly due to a lack of homology to existing structures), but their predictions are displayed to indicate their approximate structure. Extracellular domains of autotransporters have only been shown where accurate models could be built or crystal structures were available. OMPs are grouped here by the number of β-strands and then by protein family. The non-OMP subunits of the BAM complex are labeled below the central BamA subunit. Protein names are in red if they represent pseudogenes (inactivated by mutation in this strain) and blue if they are encoded on the F plasmid. The color of the box surrounding the protein names represents the number of β-strands in the β-barrel. Light Orange, 8; red, 10; light blue, 12; violet, 14; pink, 16; purple, 18; light green, 22; dark green, 24; brown, 26; black, oligomeric split β-barrel; gray, α-helical transmembrane region. Structures were aligned with each other by their β-barrel domains and rendered individually in PyMOL 2.X (Schrödinger, LLC). A list of the proteins with their associated family and PDB code can be found in Table S1.
