Figure 6.
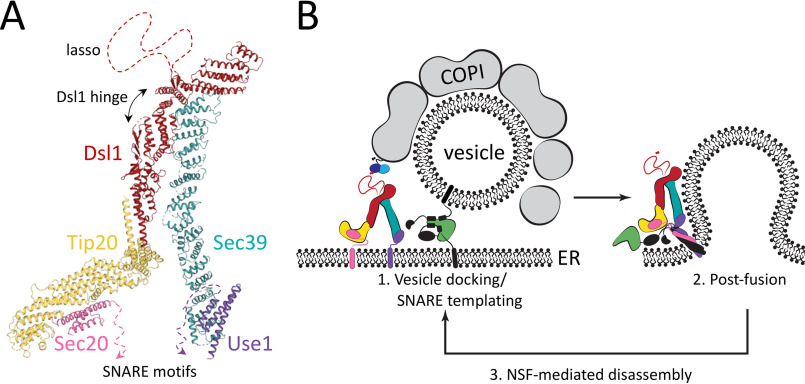
Proposed model for Dsl1 complex–mediated COPI vesicle tethering and fusion. A, a composite model of the complete Dsl1 complex in complex with the ER SNAREs Sec20 and Use1 was generated by combining the current structures with previously reported structures of S. cerevisiae Dsl1 and Tip20 (PDB accession codes 3FHN and 3ETV). At the base of the complex, the two SNARE motifs can extend toward one another and toward the ER membrane. At the top of the complex are the COPI-interacting lasso and the Dsl1 flexible hinge. B, the Dsl1 complex, colored as in A, can adopt a range of conformations, positioning Sec20 and Use1 close enough to enter into the same SNARE bundle (right) or at a distance. Incoming vesicles are tethered by the Dsl1 lasso, and at later stages, a membrane-bridging complex is formed by the SM protein Sly1 (green) binding to SNAREs on the vesicle and ER membrane. The Dsl1 complex may remain associated with the same SNAREs through multiple cycles of SNARE assembly and Sec17/18–mediated disassembly.