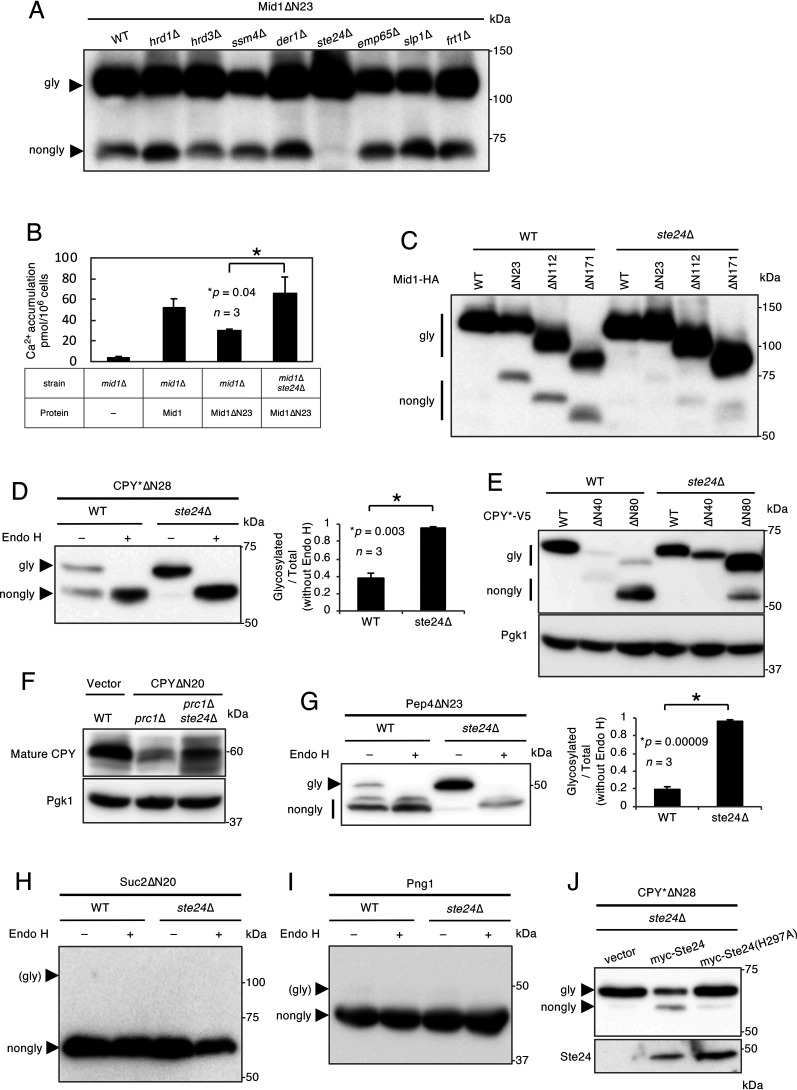
Figure 1.
Mid1ΔN23, CPY*ΔN28, and Pep4ΔN23 are translocated into the ER. A, Western blot analysis of Mid1ΔN23. Mid1ΔN23 tagged C terminally with the HA antigen was expressed in cells of the WT (strain H207) and indicated isogenic mutants. Extracts from these cells were resolved by SDS-PAGE, and Mid1ΔN23 was visualized by immunoblotting using an anti-HA antibody. B, measurement of Ca2+ accumulation. Ca2+ accumulation in mid1Δ (strain H311) cells bearing an empty vector or the Mid1 or Mid1ΔN23 plasmid or mid1Δ ste24Δ cells bearing the Mid1ΔN23 plasmid was measured using 45CaCl2. n represents the number of biological replicates. C, Western blot analysis of N-terminally truncated mutants of Mid1. Mid1, Mid1ΔN23, Mid1ΔN112, and Mid1ΔN171 were expressed in WT and ste24Δ cells (H207 background). Extracts from these cells were resolved by SDS-PAGE, and proteins were visualized by immunoblotting using an anti-HA antibody. D, Endo H treatment of CPY*ΔN28. CPY*ΔN28 tagged C terminally with the V5 antigen was expressed in WT and ste24Δ cells. Cell extracts were mock treated (−) or digested (+) with Endo H and resolved by SDS-PAGE. CPY*ΔN28 was visualized by immunoblotting using an anti-V5 antibody. The bar graph shows the relative ratio of the glycosylated versus total CPY*ΔN28 proteins. n represents the number of biological replicates. E, Western blot analysis of N-terminally truncated mutants of CPY*. CPY*, CPY*ΔN40, and CPY*ΔN80 were expressed in WT and ste24Δ cells. Extracts from these cells were resolved by SDS-PAGE, and the proteins were visualized by immunoblotting using an anti-V5 antibody. The immunoblot was also probed with an anti-Pgk1 antibody as a loading control. F, Western blot analysis of endogenous CPY and CPYΔN20. CPYΔN20 tagged C terminally with the V5 antigen was expressed in prc1Δ and prc1Δ ste24Δ cells. Cell extracts were resolved by SDS-PAGE. Endogenous CPY and CPYΔN20 were visualized by immunoblotting using an anti-CPY antibody. The immunoblot was also probed with an anti-Pgk1 antibody as a loading control. G, Endo H treatment of Pep4ΔN23. Pep4ΔN23 tagged C terminally with the DYKDDDDK epitope was expressed in WT and ste24Δ cells. Cell extracts were mock treated (−) or digested (+) with Endo H and resolved by SDS-PAGE. Pep4ΔN23 was visualized by immunoblotting using anti-DYKDDDDK antibody. The bar graph shows the relative ratio of the glycosylated versus total Pep4ΔN23 proteins. n represents the number of biological replicates. H, Endo H treatment of Suc2ΔN20. Suc2ΔN20 tagged C terminally with the V5 antigen was expressed in WT and ste24Δ cells. Cell extracts were mock treated (−) or digested (+) with Endo H and resolved by SDS-PAGE. Suc2ΔN20 was visualized by immunoblotting using an anti-V5 antibody. I, Endo H treatment of Png1. Png1 tagged C terminally with the V5 antigen was expressed in WT and ste24Δ cells. Cell extracts were mock treated (−) or digested (+) with Endo H and resolved by SDS-PAGE. Png1 was visualized by immunoblotting using an anti-V5 antibody. J, Complementation assay for Ste24. Ste24 or its protease activity-less mutant, Ste24(H297A), N-terminally tagged with the myc epitope, was coexpressed with CPY*ΔN28-V5 in ste24Δ cells. Extracts from these cells were resolved by SDS-PAGE, and proteins were visualized by immunoblotting using anti-myc and -V5 antibodies.