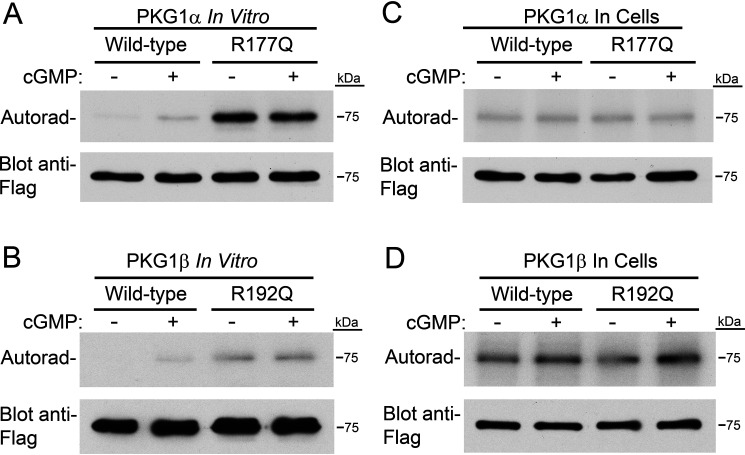
Figure 2.
The RQ mutation causes higher autophosphorylation in vitro but not in cells. A, in vitro PKG1α autophosphorylation. Purified PKG1α was incubated for 5 min with 32PO4–γ-ATP under reaction conditions identical to those used for in vitro kinase assays (in the absence of peptide substrate). Phosphate incorporation was determined by SDS-PAGE/autoradiography. Equal loading of the kinase is shown by Western blotting with an anti-Flag antibody. B, performed as in panel A, except using purified PKG1β. C, 293T cells were transfected with expression vectors for Flag-tagged WT and R177Q PKG1α. Six hours posttransfection, cells were incubated with 32PO4 for three hours, and then some cells were treated with 8-pCPT–cGMP for one hour. Upper, PKG was isolated by anti-FLAG immunoprecipitation, and phosphate incorporation was analyzed by SDS-PAGE/autoradiography. Lower, equal PKG amounts were determined by anti-Flag Western blots. D, autophosphorylation of PKG1β in 293T cells performed as in panel C.