Figure 4.
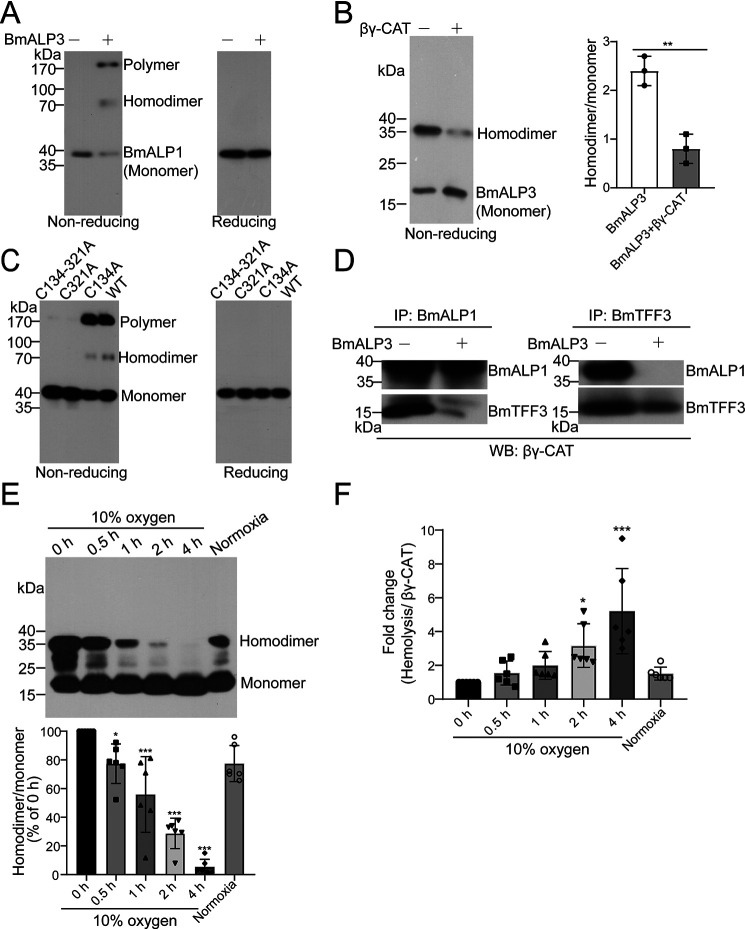
BmALP3 inhibits βγ-CAT by triggering the oxidation of BmALP1 and the dissociation of βγ-CAT. A, βγ-CAT was first incubated with BmALP3. Then, the state of BmALP1 was detected by Western blotting using an anti-BmALP1 antibody in nonreducing and reducing SDS-PAGE. B, βγ-CAT was first incubated with BmALP3. Then, the BmALP3 homodimer and monomer were detected by Western blotting using an anti-BmALP3 antibody in nonreducing SDS-PAGE (left), and the bands were semiquantified with ImageJ (right). The data represent the mean ± S.D. of triplicate samples. **, p < 0.01 by unpaired t test. C, recombinant rBmALP1 (WT) and rBmALP1 mutants were first incubated with BmALP3. Then, the state was detected by Western blotting using an anti-BmALP1 antibody in nonreducing SDS-PAGE. D, βγ-CAT was first incubated with BmALP3. Then, immunoprecipitation (IP) was performed with anti-BmALP1 and anti-BmTFF3 antibodies, and detection was performed by Western blotting (WB) using an anti-βγ-CAT antibody. E and F, toads were exposed to hypoxic conditions (10% oxygen) for different times and recovered under normal oxygen conditions (20.95%). The toad skin secretions were collected and diluted to 1 mg/ml. The BmALP3 homodimer and monomer were detected by Western blotting (top) and semiquantified with ImageJ (bottom) (E). The specific hemolytic activity of βγ-CAT in skin secretions was also detected (F). The data represent the mean ± S.D. from 6 toads. *, p < 0.05; ***, p < 0.001 by ANOVA with post hoc contrasts by Dunnett's multiple-comparison test. All of the data are representative of at least two independent experiments.