Figure 2.
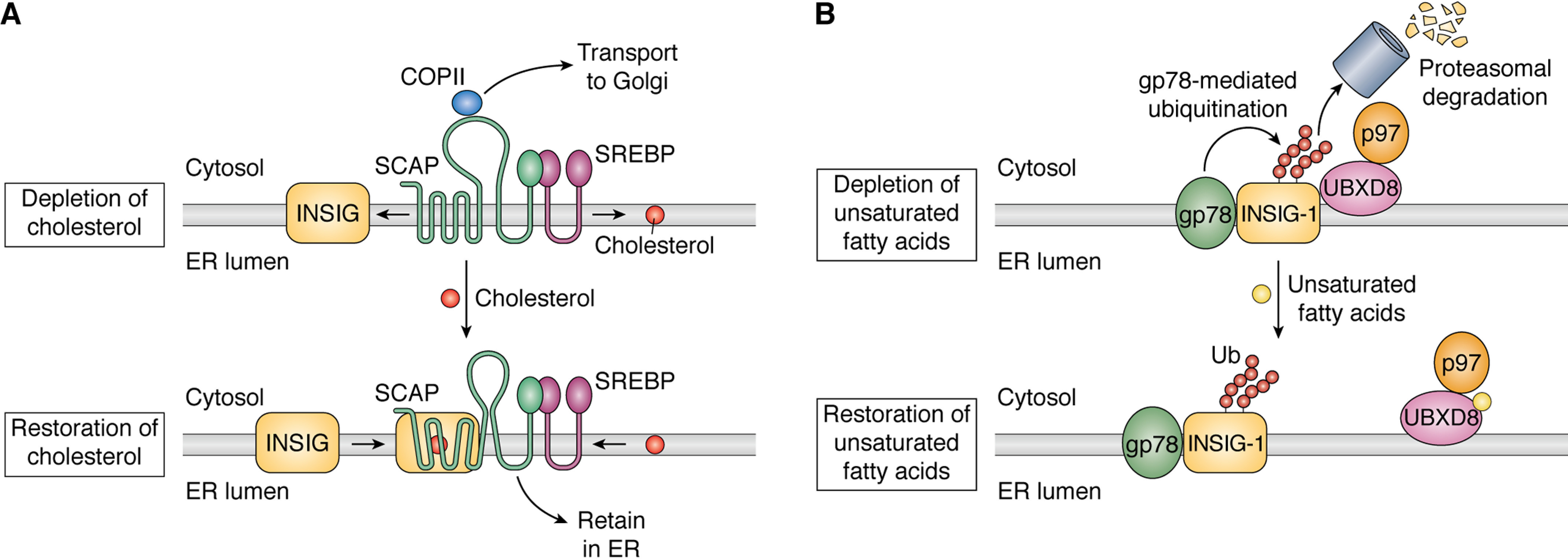
Cholesterol and unsaturated fatty acid–regulated RIP of SREBP. A, in cholesterol-depleted cells, SCAP is dissociated from INSIG proteins and cholesterol. SCAP under this condition interacts with components of COPII, allowing the SCAP/SREBP complex to be incorporated into COPII-coated vesicles so that SREBP is delivered to Golgi for proteolytic activation by S1P and S2P. In cells replete with cholesterol, interaction with cholesterol causes SCAP to bind INSIG proteins. This interaction leads to a conformational change in SCAP that inhibits its incorporation into the COPII-coated vesicles. The SCAP/SREBP complex is thus retained in the ER, preventing proteolytic activation of SREBP. B, in cells depleted of unsaturated fatty acids, INSIG-1 is ubiquitinated by gp78 and interacts with UBXD8, a p97-associated protein. The ubiquitination and p97 recruitment cause INSIG-1 to be rapidly degraded by proteasomes. In cells replete with unsaturated fatty acids, the fatty acids bind to UBXD8, leading to dissociation of UBXD8 from INSIG-1. In the absence of UBXD8-mediated recruitment of p97, INSIG-1 is stabilized, making cholesterol more effective in inhibiting proteolytic activation of SREBP.
