Figure 3.
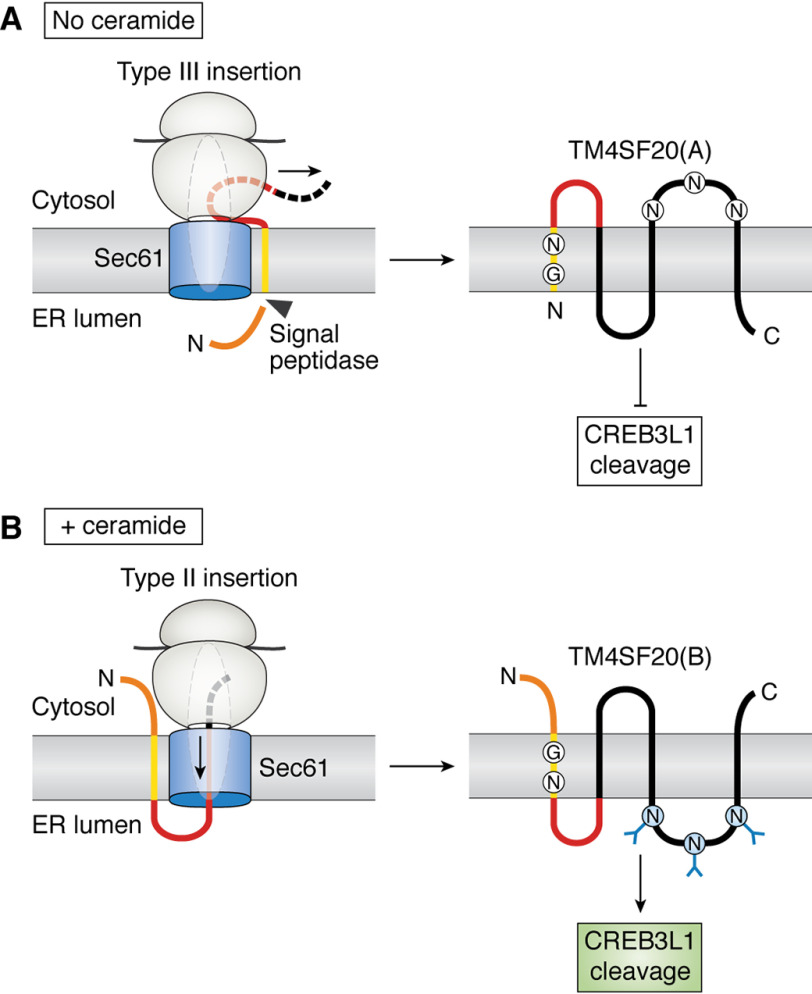
Ceramide-induced RAT of TM4SF20. A, in the absence of ceramide, the first transmembrane helix of TM4SF20 is translocated by the type III insertion mechanism. This type of insertion causes the sequence N-terminal to the transmembrane helix to be translocated into the ER lumen through an unknown mechanism, allowing it to be cleaved by signal peptidase. The nascent peptide C-terminal to the first transmembrane helix is synthesized in the cytosol. This type of translocation produces TM4SF20(A). This form of the protein is not glycosylated because the loop containing the three potential N-linked glycosylation sites is located in cytosol. TM4SF20(A) inhibits RIP of CREB3L1. B, in the presence of ceramide, the first transmembrane helix of TM4SF20 is translocated by the type II insertion mechanism, during which the nascent peptide C-terminal to the transmembrane helix is pushed through the Sec61 translocon by the ribosome and embedded into the ER lumen. This type of translocation produces TM4SF20(B), in which the sequence N-terminal to the transmembrane helix remains intact in the cytosol. TM4SF20(B) is glycosylated and activates RIP of CREB3L1. A and B, the N-terminal loop, the first transmembrane helix, the loop between the first and second transmembrane helix, and the rest of TM4SF20 are highlighted in orange, yellow, red, and black, respectively. The arrow and dashed lines indicate the direction where nascent polypeptide is extended.