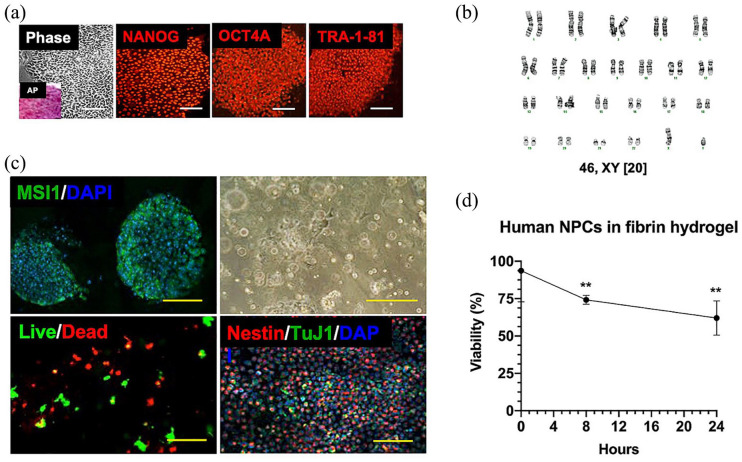
Figure 2.
Neural progenitor hydrogel patch from human-induced pluripotent stem cells (iPSCs). (a) Representative colony morphology under phase microscopy with alkaline phosphatase (AP, inset) staining of iPSCs derived from amniotic fluid (left panel), and immunofluorescence profile of iPSCs using antibodies against several markers of pluripotency, including NANOG, OCT4A, and TRA-1-81 (middle and right panel, Cy3 secondary, magnification: 20×). Scale bars represent 100 mm. (b) Karyotype analysis of representative neural progenitor cells (NPCs; passage 8) derived from a male MMC fetus revealed 46,XY and no clonal aberrations. (c) Differentiation of iPSCs into neural progenitors as shown by immunofluorescent staining of neuroepithelial rosettes for musashi-1 (MSI1, FITC secondary, magnification: 40×, left upper panel). Nuclei were counterstained with DAPI. Scale bar represents 50 mm. Representative brightfield microscopy appearance of neural progenitors resuspended in fibrin hydrogel patches (magnification: 10×, right upper panel). Scale bar represents 200 mm. Demonstration of >60% 24-h NPC viability within hydrogel patch based on intracellular esterase activity (calcein-AM green) and plasma membrane integrity (ethidium homodimer-1 red, magnification: 40×, left lower panel). Scale bar represents 100 mm. Phenotypic characterization of neural progenitors by immunofluorescent staining showing abundant nestin-positive cells (Cy3 secondary) within hydrogel patches at 2 h. There was a relative paucity of TuJ1-positive cells (FITC secondary, magnification: 10×, right lower panel). Nuclei were counterstained with DAPI. Scale bar represents 500 mm. (d) Viability of human NPCs over time after resuspension in fibrin hydrogels based on intracellular esterase activity and plasma membrane integrity. Data are presented as mean ± SEM, **p ⩽ 0.01 (Kruskal–Wallis compared to time 0), n = 4 independent biological replicates.