Abstract
For most adolescent and young adult (AYA) cancers, age‐specific molecular features are poorly understood. EORTC‐SPECTA, an academic translational research infrastructure for biomaterial collection, will explicitly recruit AYA patients and will therefore collect empirical data to bridge the molecular gap between pediatric and adult oncology. The initial pilot study, activated in February 2019 across Europe, will recruit 100 AYA patients (aged 12–29 years) with newly diagnosed or relapsed high‐grade gliomas and high‐grade bone and soft tissue sarcomas. The primary objective of the pilot is to determine feasibility and recruitment rates. Formalin‐fixed tumor tissue and whole blood from study participants will be prospectively collected with clinical data and stored centrally at the Integrated BioBank of Luxembourg. Whole exome sequencing of matched tumor and blood, and tumor RNA sequencing and DNA methylation profiling will be performed at the German Cancer Research Center, Heidelberg, Germany. Virtual central pathology review of scanned diagnostic slides will be undertaken by an international expert panel, and diagnostic material returned to the participating centers. A multidisciplinary molecular tumor board will release a clinically validated report to referring clinicians within 4–6 weeks after biopsy. SPECTA‐AYA constitutes a major opportunity to gain knowledge about the tumor biology of this unique age group. It incorporates notable innovative aspects: AYA specificity, pan‐European academic collaboration, centralized biobanking, comprehensive molecular profiling and virtual central pathology review, among others. SPECTA‐AYA will help untangle the tumor particularities of AYAs with cancer and aims to improve their access to novel drugs and personalized medicine.
Keywords: adolescents and young adults, molecular profiling, personalized medicine, high grade glioma, sarcoma
Short abstract
What's new?
To date, age‐specific molecular features remain poorly understood for most adolescent and young adult (AYA) cancers. This paper presents how SPECTA, a pan‐European academic translational research infrastructure for biomaterial collection, will specifically recruit AYA patients to bridge the molecular gap between pediatric and adult oncology. Further notable innovative aspects include centralized biobanking, comprehensive molecular profiling, and virtual central pathology review. SPECTA‐AYA, whose initial pilot study was launched in February 2019, constitutes a major opportunity to gain knowledge about the tumor biology of this unique age group and aims to improve the access of AYAs to novel drugs and personalized medicine.
Introduction: Research in Context
In Europe, over 66,000 adolescents and young adults (AYAs) aged 15–39 are diagnosed annually with cancer, from an estimated AYA population of just over 50 million.1 All cancers are rare in this age group: either rare in absolute terms or rare examples of common cancers occurring outside of the usual age range. Overall survival across the age cohort exceeds 80% at 5 years; however, among 15–24 years aged AYAs, cancer is the leading disease‐related cause of death with at least 2,000 deaths annually. For some of the more common cancer diagnoses among AYAs such as acute leukemia, certain high‐grade gliomas and bone and soft tissue sarcoma, AYA outcomes are worse than for children with the same cancers,2 and there has been little progress in improving survival figures. The molecular era brings hope of survival improvement for these historically treatment‐resistant malignancies, but greater understanding of both cancer and patient biology will be needed to achieve that improvement.1, 3, 4
There is evidence, from some of the most comprehensively studied cancers that occur throughout this age group, that the frequency of specific molecular features changes significantly with age: BCR‐ABL1 positivity is more common and good‐risk molecular features, such as high hyperdiploidy and ETV6‐RUNX1 fusions, less common in the AYA population with acute lymphoblastic leukemia than in children; Sonic‐Hedgehog (SHH) subgroup medulloblastomas account for a larger proportion of adults with medulloblastoma and SHH pathway mutations are more likely to occur upstream in the pathway than in children and childhood glioblastoma is more likely to involve H3F3A mutations and less likely to involve TERT mutations than in adults. For most AYA cancers, however, age‐specific molecular features are poorly understood.5
The role of germline predisposition is well described in pediatric cancers and in some young adult populations such as women with early onset breast cancer.6 Such mutations are most likely to be relevant for young patients. While there has been increasing recognition of previously undiagnosed cancer predisposition syndromes in young children, detailed reports of clinical phenotype in patients with well‐described clinical entities such as the neurofibromatoses (NF1 or NF2 mutation) and Li–Fraumeni syndrome (TP53 mutation),7 the prevalence of predisposing germline mutations in AYA cancer is unknown.
Several large‐scale genomic profiling initiatives are underway across Europe for childhood and adult cancers (e.g., the INFORM, MASTER, MAPPYACTS, SPECTA platforms), either for molecular screening for specific clinical trials or as stand‐alone enterprises.8, 9, 10 These initiatives share the common aims of understanding individual molecular drivers of tumorigenesis and predicting response to treatment. However, none have been designed specifically to recruit AYA patients and, therefore, recruitment of AYA patients to these initiatives is likely to be fragmentary.
In general, clinical trial recruitment rates of AYAs are poor, both for early and late‐phase trials; a situation that multi‐stakeholder initiatives such as the ACCELERATE platform are advocating to overturn.11, 12 Most clinical trials are designed to study either children or adults, have arbitrarily defined age eligibility criteria and directly or indirectly exclude many AYA patients.13 Such trials tend to recruit poorly from the AYA population.12 Since the majority of modern‐era early and late‐phase trials include biological study components, failure to recruit AYA patients to trials also results in their biology being relatively understudied compared to their “child” or “adult” counterparts.
For this reason, SPECTA will explicitly recruit AYA patients and will therefore collect empirical data to bridge the molecular gap between pediatric and adult oncology. Because SPECTA will analyze tumor and germline DNA (to assess if the mutation is hereditary or somatic), this project will describe the frequency of specific cancer‐causing germline mutations across the spectrum of AYA cancers. This may be of particular interest among the population of young patients who develop cancers typically found in older adult populations.
The initial pilot study, activated in February 2019, will be composed of 100 AYA patients with high‐grade gliomas and high‐grade bone and soft tissue sarcomas, the two cancer groups responsible for most deaths in this age group. The primary objective of the pilot is to determine feasibility and recruitment rates of eligible AYA cancer patients who consent to genetic analysis of their tumor and constitutional DNA.
Methods: Study Design and Workflow
SPECTA is a collaborative, pan‐European translational research infrastructure that enables biomaterial collection for patients treated in clinical trials or per standard of care. In SPECTA‐AYA, patients aged between 12 and 29 years with newly diagnosed, or any relapsed high‐grade glioma, including cases with malignant progression from lower‐grade tumors, and nongrade 1 bone or soft tissue sarcoma will be recruited. Formalin‐fixed tumor tissue and whole blood from study participants will be prospectively collected with clinical data.
The SPECTA‐AYA project is being carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All procedures are and will be performed in compliance with the laws and regulations of each participating country, and the appropriate institutional ethics committee(s) have approved them as required by the applicable national legislation. Informed consent and assent is being obtained for all patients or legal representatives according to the age and to the legal requirements of each country. Particular attention is given in the information sheets and informed consent forms to the implications of genetic testing, especially in regard to profiling of the germline and to the management of incidental findings.
Funds from the global EORTC‐SPECTA were allocated to this initial pilot project. As for the expanded cohorts foreseen to be started in SPECTA‐AYA after this pilot, we believe the prospect of securing funding to be fairly good, given the growing interest by multi‐stakeholder platforms to invest in precision medicine initiatives, especially for rare cancers.
The study workflow is shown in Figure 1. The biological samples will be stored centrally and nucleic acids extracted at the Integrated BioBank of Luxembourg (IBBL). Whole exome sequencing (WES) of matched tumor and blood, and tumor RNA sequencing and DNA methylation profiling (EIPC array, Illumina) will be performed at the German Cancer Research Center (DKFZ), Heidelberg. The genomic data will be deposited on the European Genome‐Phenome Archive.14 Virtual central pathology review of scanned diagnostic slides will be undertaken by an international expert panel, and diagnostic material returned to the participating centers. A multidisciplinary molecular tumor board, held monthly, will release a clinically validated report that we aim to be available to referring clinicians within 4 to 6 weeks after biopsy. Leftover material from the collected formalin‐fixed tumor tissue and blood will be stored for future research.
Figure 1.
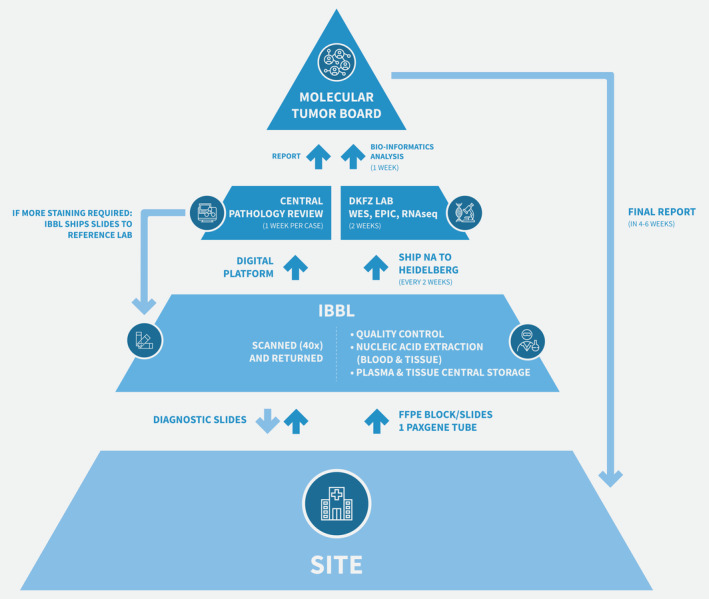
Study workflow.
Discussion: Innovative Aspects
SPECTA‐AYA is an ambitious, European collaborative project that includes several innovative aspects, which ought to be highlighted.
While there are several ongoing molecular profiling initiatives across Europe for children and adults with cancer, SPECTA‐AYA is the first comprehensive, international platform specifically targeted to AYA patients. AYAs with cancer constitute a unique age group with distinct features regarding epidemiology, tumor biology, inclusion rates into clinical trials, clinical outcomes and psychosocial setting.5, 15, 16, 17 SPECTA‐AYA will help decipher the tumor peculiarities of this group of patients and aims to improve their access to novel drugs and personalized medicine.
Scarcity of available tumor material and a preponderance of formalin‐fixed over frozen tissue are recognized to be major challenges of translational research18 in rare populations such as this study population. Collection and storage of frozen tissue is increasing with time for childhood cancers and is viewed as standard of care in some countries, but it is not the norm for AYA patients in general or for the histologies included in SPECTA‐AYA specifically. To understand better the biology of AYA cancers, in this study, we aim to utilize nucleic acids from blood and from the paraffin‐embedded tissue almost universally available from diagnostic biopsies to generate a complete molecular profile for each patient. RNA sequencing, where quality permits, will be used to identify gene fusions and gene expression, WES will identify numerical and sequence variants, tumor mutation burden and microsatellite instability calculation and, finally, methylation array will be used to validate the tumor classification19, 20, 21 and inform copy number variations. Germline DNA will be used as control for WES and to assess predisposition syndromes, as described in Reference 22. Finally, circulating tumor DNA will also be extracted from blood samples and stored for future research.
Another hurdle in this rare patient population is the need for central pathology review. In SPECTA‐AYA, a virtual pathology review platform has been developed. After patient registration, diagnostic slides (H&E and selected stainings) will be sent to IBBL, scanned at 40× magnification and uploaded onto the telepathology platform AGOKO23 with associated clinical data. Immunostained slides will be returned to the recruiting site. An international panel of expert pathologists will review each case and make a consensus pathological diagnosis according to an agreed minimal histopathological and immunohistochemical marker set. Additional stains, if required, will be performed at a central laboratory. A comparison with the methylation classifier20 will also be performed and will contribute to the agreed final diagnosis. A certain degree of overlap in the molecular analyses performed locally as part of initial diagnosis can be expected and may be instructive, as for instance, some molecular features tested by immunohistochemistry in the local pathology laboratory can be corroborated by the techniques applied in SPECTA‐AYA centrally, enhancing the power of the central pathology review. This can prove particularly relevant in this setting of rare tumors, in which the pathology diagnosis is frequently challenging.
The molecular tumor board will include expert pathologists, molecular biologists and clinicians, including the recruiting clinician, and will take place monthly, an approach that has been shown to be feasible in cancer networks.8, 10, 24 The final report released to the recruiting center will include an agreed clinical, pathological and molecular diagnosis, a rationale for molecularly targeted treatments where appropriate, and suggestions for clinical trial inclusion if available. Germline mutations, if any, and relevant incidental findings are included as well. Additionally, the clinician (on behalf of the patient and family) can request the comprehensive sequencing raw data.
The SPECTA infrastructure also allows for long‐term follow‐up and collection of additional clinical data including patient‐reported and neurocognitive outcomes. Such variables have not been selected for the AYA pilot project, but could be easily added in a follow‐up cohort.
The pan‐European nature of the project, the comprehensive molecular analysis, and the virtual central pathology review process combine into a complex workflow (Fig. 1) with subsequent operational challenges. The collaboration between three major academic cancer research organizations in Europe—the UK National Cancer Research Institute, EORTC and DKFZ—has been a major facilitator in mastering these challenges. Furthermore, while decentralized storage greatly reduces the chances of prompt and reliable translational research, the involvement of IBBL as a centralized solution with shared standard operating procedures to ensure best practice in biomaterial and clinical data collection is a key strength of SPECTA. The collaboration with DKFZ ensures that the latest genomic analysis and bioinformatic assessment will be performed during the course of the project. Finally, data and sample sharing policies are already in place at EORTC to promote academic collaboration and research, especially needed in the field of rare cancers. It is therefore EORTC's policy to consider for sharing all data generated from its research while safeguarding intellectual property, the privacy of patients and confidentiality.25 This is done upon request from qualified scientific and medical researchers, for reanalysis and other research purposes. EORTC complies with the OECD Principles and Guidelines for Access to Research Data from Public Funding and with the National Institutes of Health Final Statement on Sharing Research Data released on February 26, 2003; it is committed to do so within the limits needed to guarantee the protection of personal data in accordance with the EU data protection rules and other applicable European legislation.
Conclusions/Output
SPECTA‐AYA constitutes a major opportunity to gain knowledge about the tumor biology of this unique age group. It incorporates notable innovative aspects: AYA specificity, pan‐European academic collaboration, centralized biobanking, comprehensive molecular profiling and virtual central pathology review, among others. We believe that the expected challenges can be overcome with strong collaborative efforts, notably between adult and pediatric oncology, such as the DKFZ–EORTC partnership. SPECTA‐AYA will help untangle the tumor particularities of AYAs with cancer and, in the longer term, aims to improve their access to novel drugs and personalized medicine.
Acknowledgements
This publication was partially supported by Unai Kest Memorial Run. The work of T.d.R. as Fellow at EORTC Headquarters was supported by a grant from Fonds Cancer (FOCA) from Belgium. We would like to thank Laurence Decroix and Frederic Rince, both members of the EORTC Communication Team, for producing the workflow figure.
Conflict of Interest: M.P. has received honoraria for lectures, consultation or advisory board participation from the following for‐profit companies: Bayer, Bristol‐Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Merck Sharp & Dome. F.B. has an immediate family member employed, owns stocks or received research funding from the following for‐profit companies: Celgene, Crossject, Sanofi, Abbvie. S.F. has had a consulting or advisory role, received honoraria, research funding and/or travel/accommodation expenses funding from the following for‐profit companies: Bayer, Roche, Amgen, Eli Lilly, PharmaMar, AstraZeneca and Pfizer. W.V.D.G. has received research funding from Novartis and advisory board honoraria from Bayer. The rest of the authors have no conflict of interest to disclose.
References
- 1. Desandes E, Stark DP. Epidemiology of adolescents and young adults with cancer in Europe. Prog Tumor Res 2016;43:1–15. [DOI] [PubMed] [Google Scholar]
- 2. Chen I, Pasalic D, Fischer‐Valuck B, et al. Disparity in outcomes for adolescent and young adult patients diagnosed with pediatric solid tumors across 4 decades. Am J Clin Oncol 2018;41:471–5. [DOI] [PubMed] [Google Scholar]
- 3. Sundar R, McVeigh T, Dolling D, et al. Clinical outcomes of adolescents and young adults with advanced solid tumours participating in phase I trials. Eur J Cancer 2018;101:55–61. [DOI] [PubMed] [Google Scholar]
- 4. McVeigh TP, Sundar R, Diamantis N, et al. The role of genomic profiling in adolescents and young adults (AYAs) with advanced cancer participating in phase I clinical trials. Eur J Cancer 2018;95:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cha S, Lee J, Shin J‐Y, et al. Clinical application of genomic profiling to find druggable targets for adolescent and young adult (AYA) cancer patients with metastasis. BMC Cancer 2016;16:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poggio F, Lambertini M, Bighin C, et al. Management of young women with early breast cancer. ESMO Open 2018;3:e000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bougeard GG, Renaux‐Petel M, Flaman J‐MM, et al. Revisiting Li‐Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol 2015;33:2345–52. [DOI] [PubMed] [Google Scholar]
- 8. Worst BC, van Tilburg CM, Balasubramanian GP, et al. Next‐generation personalised medicine for high‐risk paediatric cancer patients – the INFORM pilot study. Eur J Cancer 2016;65:91–101. [DOI] [PubMed] [Google Scholar]
- 9. Harttrampf AC, Lacroix L, Deloger M, et al. Molecular screening for cancer treatment optimization (MOSCATO‐01) in pediatric patients: a single‐institutional prospective molecular stratification trial. Clin Cancer Res 2017;23:6101–12. [DOI] [PubMed] [Google Scholar]
- 10. Horak P, Klink B, Heining C, et al. Precision oncology based on OMICS data: the NCT Heidelberg experience. Int J Cancer 2017;141:877–86. [DOI] [PubMed] [Google Scholar]
- 11. Vassal G, Rousseau R, Blanc P, et al. Creating a unique, multi‐stakeholder paediatric oncology platform to improve drug development for children and adolescents with cancer. Eur J Cancer 2015;51:218–24. [DOI] [PubMed] [Google Scholar]
- 12. Gaspar N, Marshall LV, Binner D, et al. Joint adolescent‐adult early phase clinical trials to improve access to new drugs for adolescents with cancer: proposals from the multi‐stakeholder platform‐ACCELERATE. Ann Oncol 2018;29:766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Rojas T, Neven A, Terada M, et al. Access to clinical trials for adolescents and young adults with cancer: A meta‐research analysis. JNCI Cancer Spectr [Internet]. Available from: http://fdslive.oup.com/www.oup.com/pdf/production_in_progress.pdf (accessed Aug 21, 2019). [DOI] [PMC free article] [PubMed]
- 14. EGA European Genome‐Phenome Archive [Internet]. Available from: https://ega-archive.org/ (accessed Jul 9, 2019).
- 15. Tai E, Buchanan N, Westervelt L, et al. Treatment setting, clinical trial enrollment, and subsequent outcomes among adolescents with cancer: a literature review. Pediatrics 2014;133:S91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chuk MK, Mulugeta Y, Roth‐Cline M, et al. Enrolling adolescents in disease/target‐appropriate adult oncology clinical trials of investigational agents. Clin Cancer Res 2017;23:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stark D, Bielack S, Brugieres L, et al. Teenagers and young adults with cancer in Europe: from national programmes to a European integrated coordinated project. Eur J Cancer Care (Engl) 2016;25:419–27. [DOI] [PubMed] [Google Scholar]
- 18. Rutkowski S, Modena P, Williamson D, et al. Biological material collection to advance translational research and treatment of children with CNS tumours: position paper from the SIOPE brain tumour group. Lancet Oncol 2018;19:e419–28. [DOI] [PubMed] [Google Scholar]
- 19. Gómez S, Garrido‐Garcia A, Garcia‐Gerique L, et al. A novel method for rapid molecular subgrouping of medulloblastoma. Clin Cancer Res 2018;24:1355–63. [DOI] [PubMed] [Google Scholar]
- 20. Capper D, Jones DTW, Sill M, et al. DNA methylation‐based classification of central nervous system tumours. Nature 2018;555:469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MolecularNeuropathology [Internet]. Available from: https://www.molecularneuropathology.org/mnp (accessed Apr 9, 2019).
- 22. Waszak SM, Northcott PA, Buchhalter I, et al. Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol 2018;19:785–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agoko ‐ Solutions in TelePathology [Internet]. Available from: https://www.agoko.be/index.html (accessed Mar 29, 2019).
- 24. Rolfo C, Manca P, Salgado R, et al. Multidisciplinary molecular tumour board: a tool to improve clinical practice and selection accrual for clinical trials in patients with cancer. ESMO Open 2018;3:e000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EORTC. Data sharing ‐ EORTC [Internet]. Available from: https://www.eortc.org/data-sharing/ (accessed Jul 9, 2019).


