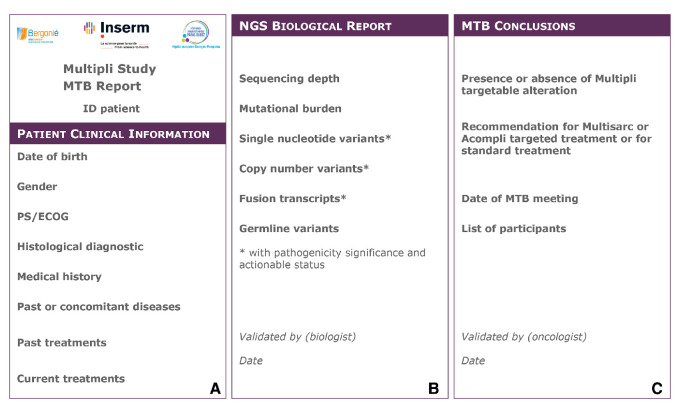
Figure 4.
The Multipli Molecular Tumour Board (MTB) Report. For each Single Nucleotide Variant, gene access number (NM), Human Genome Variation Society (HGVS) nomenclature, level of pathogenicity and actionability and eventual association with a loss of heterozygosity have to be reported. For each Copy Number Variant, segment size, copy numbers, level of pathogenicity and actionability have to be reported. For each Fusion Transcript, nomenclature, breakpoint, consequence on open reading frame, level of pathogenicity and actionability have to be reported. For each Germline Variant reported, gene access number (NM), HGVS nomenclature, zygosity and level of pathogenicity have to be detailed. Drug toxicity associated variants with a 1A or 1B level of evidence (based on PharmGKB data) are reported. ID patient, patient identification number;MTB, Molecular Tumour Board; NGS, next-generation sequencing; PS/ECOG:Performance Status on the ECOG (Eastern Cooperative Oncology Group) scale.