FIGURE 1.
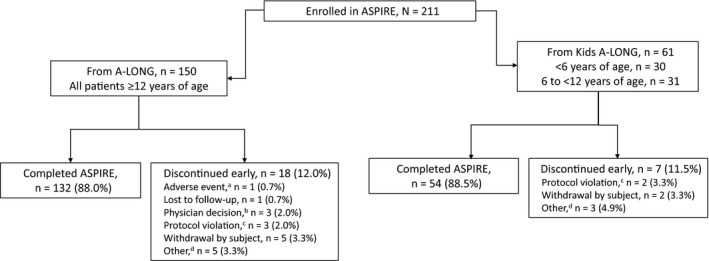
Subject disposition for ASPIRE extension study. aSubject was on an episodic treatment regimen and discontinued owing to a non‐serious adverse event of chronic renal failure that was considered unrelated to recombinant factor VIII Fc fusion protein. bSubjects were withdrawn because of the physician's decision for non‐compliance with the study (n = 3). cProtocol violations included non‐compliance with prophylactic dosing (n = 1), use of non‐study factor VIII under circumstances that were not an emergency or an accident (n = 2), non‐compliance with study procedures, including infusion timing and concomitant medications (n = 1), lost to follow‐up and incomplete end of study visit (n = 1). dIncludes product becoming commercially available in the subject's country (n = 3), commencing a different clinical trial (n = 2), inability to comply with the demands of the study (n = 1), early termination (n = 1) and incarceration (n = 1)
