Abstract
Background
Partner and localizer BRCA2 (PALB2) is a breast cancer predisposition gene, but the clinical relevance of PALB2 germline mutations in Chinese patients with breast cancer remains unknown. This study attempted to investigate the full prevalence and spectrum of PALB2 germline mutations in China and the associations between PALB2 germline mutations and breast cancer risk.
Methods
A total of 21,216 unselected patients with breast cancer were enrolled from 10 provinces in China, and 5890 Chinese women without cancer were enrolled as healthy controls. PALB2 screening was based on next‐generation sequencing.
Results
A total of 16,501 BRCA1/2‐negative patients with breast cancer were analyzed. Deleterious PALB2 mutation carriers accounted for 0.97% (n = 160) in the breast cancer cohort and for 0.19% (n = 11) in the healthy control cohort. Forty‐one novel PALB2 germline mutations were identified. A high frequency of PALB2 c.751C>T was detected, and it accounted for 10.63% of the PALB2 germline mutations detected (17 of 160). PALB2 mutations were significantly associated with increased breast cancer risk (odds ratio [OR], 5.23; 95% confidence interval [CI], 2.84‐9.65; P < .0001), especially among women 30 years old or younger (OR, 10.09; 95% CI, 3.95‐25.79; P < .0001). Clinical characteristics, including a family history, bigger tumor size, triple‐negative breast cancer, positive lymph nodes, and bilateral breast cancer, were closely related to PALB2 mutations.
Conclusions
This study revealed a comprehensive spectrum of PALB2 germline mutations and characteristics of PALB2‐related breast cancer in China. PALB2 germline mutations confer a moderately increased risk for breast cancer but profoundly increase breast cancer risk for those 30 years old or younger in the Chinese population.
Keywords: breast cancer risk, clinical characteristics, germline mutation, next‐generation sequencing, partner and localizer BRCA2 (PALB2)
Short abstract
This study reveals a comprehensive spectrum of PALB2 germline mutations and characteristics of PALB2‐related breast cancer, including the PALB2‐related breast cancer risk and distinctive clinical characteristics. Clinically, this study will provide solid evidence for the genetic counseling of patients with breast cancer and healthy women with PALB2 germline mutations, especially in the Chinese population and among those with Chinese ethnicities worldwide.
Introduction
Partner and localizer BRCA2 (PALB2) is an important DNA repair gene that is essential for its function in homologous recombination. In the DNA damage response, PALB2 links BRCA1 and BRCA2 1 and implements the recombinational repair and checkpoint functions of BRCA2 in maintaining genome integrity. 2 , 3
Germline mutations in the PALB2 gene confer a predisposition to breast cancer. 4 , 5 Several studies in familial breast cancer have found a substantially increased risk of breast cancer in PALB2 mutation carriers. 5 , 6 , 7 , 8 , 9 However, because of the low frequency of PALB2 mutations in women, we still lack data for the prevalence and spectrum of PALB2 in unselected patients with breast cancer. A study in Poland investigated 2 recurrent PALB2 mutations in a large cohort of unselected patients with breast cancer, and it confirmed an increased risk of breast cancer for carriers of these 2 recurrent PALB2 mutations. 10 Limited studies of PALB2 germline mutations have been conducted in Chinese women. Because of the possibility of differences in PALB2 mutations among different ethnic groups, it is of interest to establish the full prevalence and spectrum of PALB2 germline mutations in Chinese women.
In this study, we sequenced PALB2 genes of 21,216 unselected patients with primary breast cancer and 5890 healthy women in the Chinese population. We attempted to investigate the frequencies of PALB2 germline mutations in Chinese women with and without breast cancer to depict the full spectrum of PALB2 mutations, to assess the odds ratio (OR) for breast cancer associated with a PALB2 germline mutation, and to explore the effects of PALB2 mutations on the clinical outcomes of patients with breast cancer.
Materials and Methods
Study Population
We recruited a total of 21,216 unselected patients with primary breast cancer between 2015 and 2018 from 10 provinces in China (Supporting Table 1). The recruited patients were diagnosed from 2000 to 2017. Among these patients, 3767 were excluded because of uncertain clinical or pathological information or poor DNA quality and quantity. Nine hundred forty‐eight patients who carried deleterious BRCA1/2 germline mutations were also excluded. The remaining 16,501 BRCA1/2‐negative patients with breast cancer were analyzed. A total of 5890 healthy people (from the health examination crowd in medical centers) were further enrolled as the control group. The cohort of patients with breast cancer and healthy people in this study was a prospective cohort, and blood was collected prospectively. The ethics committee of the Second Affiliated Hospital of the Zhejiang University School of Medicine in Hangzhou (ethics approval 2015073) approved the study. All patients and healthy controls provided written informed consent. All the patients and controls will be (or have been) informed about the sequencing results.
Clinical Information Collection
Information about the age at diagnosis, tumor histology, tumor size, lymph node status, estrogen receptor status, progesterone receptor status, HER2 status, and bilaterality was abstracted from medical records. National Comprehensive Cancer Network and American Society of Clinical Oncology/College of American Pathologists guidelines were used to define the tumor characteristics. A family history of breast, ovarian, or any other cancer was collected by questionnaire. The questionnaires were handed out to patients during clinic visits. For patients with available medical records, the questionnaires about family histories were further checked against their medical records. Genetic test results from this study were considered only for research and were not used for clinical decision making. A family history of breast and/or ovarian cancer was defined as 1 or more patients with breast and/or ovarian cancer among the patient's first‐, second‐, or third‐degree relatives; a family history of any other cancer was defined as 1 or more patients with cancer (any kind of cancer except for breast and/or ovarian cancer) among the first‐ or second‐degree relatives of the patient with breast cancer.
PALB2 and BRCA1/2 Sequencing Assay
Blood samples were obtained from all patients with breast cancer and from healthy controls for DNA extraction and genotyping. All protein‐coding exons and proximal intron‐exon junctions (adjacent splice site, 10 bp) of PALB2, BRCA1, and BRCA2 were screened. The sequencing methods and analysis of the sequencing data are described in detail in our previous study. 11 , 12 Variant pathogenicity was explored on the basis of the ClinVar database and American College of Medical Genetics and Genomics recommendations. The null variants (due to nonsense, frameshift, canonical ±1 or 2 splice sites, initiation codons, or single‐exon or multi‐exon deletions) that resulted in a loss of function were classified as deleterious variants. All the pathogenic mutations were validated by Sanger sequencing. National Center for Biotechnology Information transcript NM_024675.3 was used as a reference sequence for PALB2. All PALB2 variants were named according to Human Genome Variation Society nomenclature.
Statistical Analysis
The distributions of PALB2 mutations among patients with breast cancer and healthy controls were assessed with a Pearson chi‐square test or Fisher exact test. ORs and 95% confidence intervals (CIs) were calculated with healthy controls as the reference group. Distributions of clinicopathological characteristics of patients with breast cancer and PALB2 mutations and noncarriers were compared with a Pearson chi‐square test or Fisher exact test for dichotomous variables and with a Student t test for continuous variables. Two‐sided P values less than .05 were considered statistically significant. We performed all statistical analyses with R software (version 3.5.3) unless otherwise noted.
Results
Prevalence and Spectrum of PALB2 Germline Mutations in Breast Cancer
PALB2 germline mutations were determined in our large cohort of 16,501 unselected BRCA1/2‐negative patients with breast cancer. Our study revealed that a total of 79 deleterious PALB2 germline mutations were identified in 160 of the 16,501 unselected patients with breast cancer (Fig. 1 and Supporting Table 2). The prevalence of PALB2 germline mutations was 0.97% (160 of 16,501).
Figure 1.
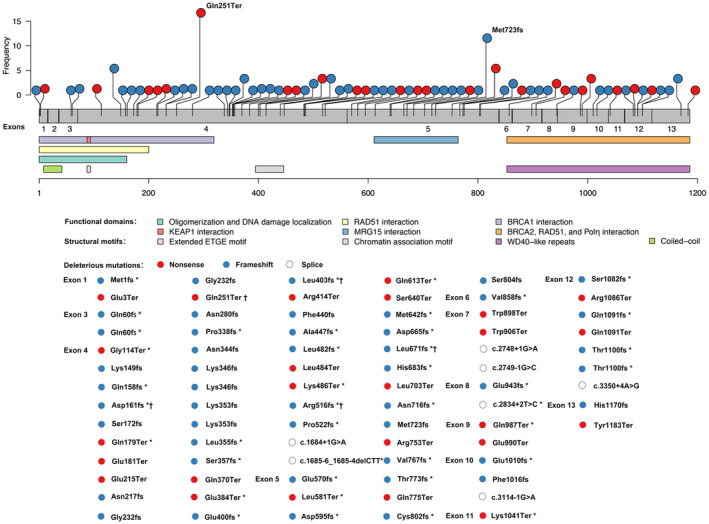
Spectrum of deleterious PALB2 mutations identified among 16,501 unselected BRCA1/2‐negative patients with breast cancer. The schematic representation of the PALB2 gene was superimposed onto the exonic structure of the gene, with structural motifs and functional domains related. The x‐axis represents the amino acid residues of the PALB2 protein. The y‐axis represents the mutation frequency. *Novel PALB2 mutations. †PALB2 mutations identified in healthy controls. PALB2 indicates partner and localizer BRCA2.
The 79 deleterious PALB2 germline mutations included 25 nonsense mutations, 47 frameshift mutations, and 7 splicing mutations (Fig. 1 and Supporting Tables 2 and 3). The hotspot regions for PALB2 mutations were exon 4 (74 of 160 [46.25%]) and exon 5 (36 of 160 [22.5%]; Fig. 2). There were 25 recurrent deleterious PALB2 germline mutations, of which the 3 most common were c.751C>T (17 of 160 [10.63%]), c.2167_2168delAT (12 of 160 [7.5%]), and c.3114‐1G>A (10 of 160 [6.25%]; Supporting Tables 2 and 3). A total of 41 novel mutations were identified in PALB2; they included 8 nonsense mutations, 31 frameshift mutations, and 2 splicing mutations (Supporting Tables 2 and 3). Most of these novel mutations were located in exon 4 (16 of 41 [39.02%]) or exon 5 (12 of 41 [29.27%]; Supporting Table 2).
Figure 2.
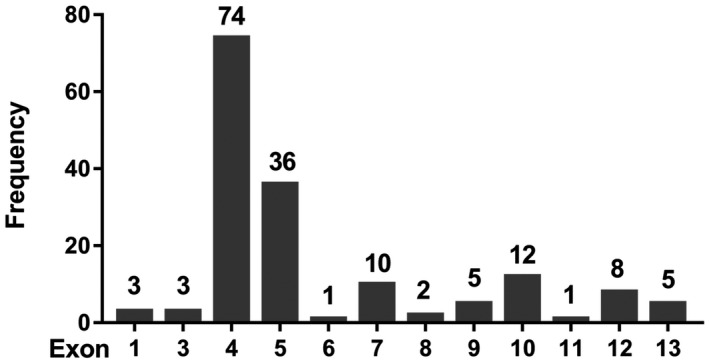
Distribution of deleterious PALB2 mutations among its exons. PALB2 indicates partner and localizer BRCA2.
PALB2 Mutations and Breast Cancer Risk
PALB2 mutations were found in 160 of 16,501 unselected BRCA1/2‐negative patients with breast cancer (0.97%) and in 11 of 5890 healthy controls (0.19%). The OR for breast cancer risk in women with a PALB2 mutation was 5.23 (95% CI, 2.84‐9.65; P < .0001; Table 1). With subdivision by age groups, the OR for breast cancer risk in women with a PALB2 mutation was 10.09 (95% CI, 3.95‐25.79; P < .0001) for those 30 years old or younger and 5.06 (95% CI, 2.74‐9.34; P < .0001) for those older than 30 years (Table 1).
Table 1.
Association of Deleterious PALB2 Mutations With Breast Cancer Risk
| Total, No. | Deleterious PALB2 Mutation Frequency, No. | Prevalence, % | OR (95% CI) | P | |
|---|---|---|---|---|---|
| Healthy controls | 5890 | 11 | 0.19 | 1.00 (reference) | |
| Patients with breast cancer | 16,501 | 160 | 0.97 | 5.23 (2.84‐9.65) | <.0001 |
| Age group | |||||
| ≤30 y | 593 | 11 | 1.85 | 10.09 (3.95‐25.79) a | <.0001 |
| >30 y | 15,883 | 149 | 0.94 | 5.06 (2.74‐9.34) | <.0001 |
Abbreviations: CI, confidence interval; OR, odds ratio; PALB2, partner and localizer BRCA2.
Fisher exact test.
PALB2 Mutations and Tumor Characteristics
There were several significant clinicopathological differences between PALB2 mutation carriers and noncarriers (Table 2). Compared with noncarriers, significantly more PALB2 mutation carriers were 30 years old or younger at diagnosis (6.88% vs 3.56%; P = .04). PALB2 mutation carriers were significantly more likely to have triple‐negative breast cancers than noncarriers (22.83% vs 13.56%; P = .004). PALB2 mutation carriers were more likely than noncarriers to have bigger tumors (tumor size ≥ 2 cm, 55.93% vs 45.93%; P = .04), positive axillary lymph nodes (lymph nodes positive, 49.60% vs 38.80%; P = .018), bilateral breast cancers (6.29% vs 2.01%; P = .003), a family history of breast and/or ovarian cancer (20.63% vs 7.96%; P < .0001), and a family history of any other cancer (23.75% vs 9.42%; P < .0001).
Table 2.
Clinicopathological Characteristics of PALB2 Mutation Carriers and Noncarriers in the 16,501 BRCA1/2‐Negative Patients With Breast Cancer
| Characteristic | Patients With Breast Cancer | PALB2 Mutation Carriers (n = 160) | PALB2 Mutation Noncarriers (n = 16,341) | P |
|---|---|---|---|---|
| Age at diagnosis | ||||
| Mean ± SD, y | 48.0 ± 10.2 | 47.4 ± 10.8 | 48.0 ± 10.2 | .52 |
| Range, n/N (%) | ||||
| ≤30 y | 593 | 11/160 (6.88) | 582/16,341 (3.56) | .04 |
| >30 y | 15,908 | 149/160 (93.12) | 15,759/16,341 (96.44) | |
| Tumor size, n/N (%) | ||||
| <2 cm | 4652 | 52/118 (44.07) | 4600/8507 (54.07) | .04 |
| ≥2 cm | 3973 | 66/118 (55.93) | 3907/8507 (45.93) | |
| Histology, n/N (%) | ||||
| Carcinoma in situ | 1016 | 6/160 (3.75) | 1010/16,341 (6.18) | .27 |
| Invasive ductal carcinoma | 14,179 | 140/160 (87.50) | 14,039/16,341 (85.91) | |
| Other invasive carcinoma | 1306 | 14/160 (8.75) | 1292/16,341 (7.91) | |
| Estrogen receptor–positive, n/N (%) | 10,111 | 92/133 (69.17) | 10,019/14,300 (70.06) | .90 |
| Progesterone receptor–positive, n/N (%) | 9135 | 81/133 (60.90) | 9054/14,310 (63.27) | .64 |
| HER2‐positive, n/N (%) | 3347 | 26/123 (21.14) | 3321/11,888 (27.94) | .12 |
| Triple‐negative breast cancer, n/N (%) | 1519 | 29/127 (22.83) | 1490/10,989 (13.56) | .004 |
| Lymph nodes positive, n/N (%) | 3415 | 62/125 (49.60) | 3353/8642 (38.80) | .018 |
| Bilateral, n/N (%) | 231 | 9/143 (6.29) | 222/11,032 (2.01) | .003 a |
| Distant metastasis, n/N (%) | 697 | 5/160 (3.13) | 692/16,341 (4.23) | .49 |
| Family history of breast and/or ovarian cancer, n/N (%) | 1331 | 33/160 (20.63) | 1298/16,311 (7.96) | <.0001 |
| Family history of any other cancer, n/N (%) | 1574 | 38/160 (23.75) | 1536/16,311 (9.42) | <.0001 |
Abbreviation: PALB2, partner and localizer BRCA2.
Fisher exact test. The significance of the bold values were less than .05, which were considered statistically significant.
Discussion
Because loss‐of‐function mutations in the PALB2 gene confer a predisposition to breast cancer, clinical genetic testing for breast cancer risk increasingly includes PALB2 in addition to BRCA1 and BRCA2. 6 Many breast cancer–related studies on PALB2 germline mutations have been conducted in European and American populations 6 , 10 , 13 ; it is important and necessary to have a detailed spectrum of PALB2 germline mutations and robust risk estimates for women who carry PALB2 germline mutations in the Chinese population.
In our study, we found that 0.97% of patients carried PALB2 germline mutations among 16,501 unselected BRCA1/2‐negative patients with breast cancer. On the basis of a rate of PALB2 mutation–positive cases of 0.19% among 5890 healthy controls, our study presents an estimated OR of 5.23 (95% CI, 2.84‐9.65; P < .0001) for breast cancer associated with a PALB2 mutation. PALB2 germline mutations are rare in breast cancer and in the general population. Thus, only a few previous studies have investigated PALB2 germline mutations in unselected breast cancer cases. Two deleterious mutations in PALB2 (509_510delGA and 172_175delTTGT) were examined in a Polish population, and that study's findings showed that the frequency of these 2 PALB2 germline mutations was 0.93% and that the estimated OR for breast cancer in PALB2 carriers was 4.39. 10 In an American population, Tung et al 14 found that the proportion with PALB2 germline mutations was 0.2% among sequential patients with breast cancer at the Dana‐Farber Cancer Institute, and the breast cancer relative risk for PALB2 carriers was 5.3. However, only 1 patient with breast cancer and a PALB2 germline mutation was identified in Tung et al's study cohort, and this indicated the limited power to estimate the penetrance and breast cancer risk of PALB2 mutations. Another study by Couch et al, 15 based on a nationwide sample population of the United States, presented a PALB2 germline mutation frequency of 0.8% among white women and an OR of 7.46 for breast cancer risk in PALB2 carriers. Data from the Chinese population are very limited. Sun et al 16 recently reported that PALB2 germline mutations accounted for 0.69% of 8085 unselected Chinese patients with breast cancer who were treated at their breast center. It was a single‐center study, and the breast cancer risk for PALB2 germline mutation carriers is still unknown. To date, the current study is the largest PALB2 mutations analysis in Chinese patients and healthy controls. The results of this study indicate that PALB2 mutations confer a moderately increased risk for breast cancer. Notably, subdivided by age group, the breast cancer risk in our study was especially high for PALB2 mutation carriers aged 30 years or younger (OR, 10.09). This underscores the necessity of regular surveillance (breast ultrasound or screening magnetic resonance imaging) for PALB2 mutation carriers, especially those aged 30 years or younger.
In agreement with previous studies of PALB2 mutations in breast cancers, 13 the hotspot region for PALB2 mutations in our study was exon 4. The most common mutation in our study, PALB2 c.751C>T (n = 17), has been reported only in Asian populations (Chinese 17 and Malaysian populations 18 ) before, and this indicates that this mutation may be enriched in Asian populations. The frequency of the PALB2 c.751C>T mutation in our unselected breast cancer cohort (17/16,501 = 0.1%) was lower than the frequency in the Chinese familial breast cancer cohort (2/360 = 0.56%) 17 or the Malaysian unselected breast cancer cohort (1/467 = 0.21%). 18 Another common mutation in our study, PALB2 c.2167_2168delAT (n = 12), has been found in 3 European hereditary breast cancer cohorts before. 6 , 19 , 20 The frequency of the PALB2 c.2167_2168delAT mutation in our unselected breast cancer cohort (12/16,501 = 0.07%) was much lower than the frequencies in the European hereditary breast cancer cohorts (UK cohort, 2/362 = 0.55% 6 ; Italian cohort 1, 1/575 = 0.17% 19 ; Italian cohort 2, 2/255 = 0.78% 20 ). Although some common PALB2 mutations previously reported in European, US, and Australian breast cancers 13 such as PALB2 c.172_175delTTGT, c.2323C>T, and c.3549C>G were detected in our study, other known and common PALB2 mutations, such as PALB2 c.196C>T, c.509_510delGA, c.1592delT, and c.3113G>A, did not occur in our study. This further underlines the variety of PALB2 mutations across different spatial distributions and ethnicities of the world.
In our study, 22.83% of the patients with breast cancer and PALB2 germline mutations (29 of 127) had a triple‐negative phenotype, whereas the frequency was 13.56% among mutation‐negative cases (P = .004). Obviously, in our study, PALB2 mutation breast cancers were significantly more likely to be the triple‐negative phenotype in comparison with noncarriers. Nevertheless, the proportion of triple‐negative breast cancers with PALB2 germline mutations in our study (22.83%) was lower than those found in previous studies (Finnish familial and sporadic breast cancers, 54% 21 ; UK hereditary breast cancer cohort, 30% 6 ; Polish unselected breast cancer cohort, 34% 10 ). In addition, PALB2 mutation carriers were more likely to have bigger, axillary lymph node–positive, and bilateral tumors, and this indicates that PALB2 mutation carriers present an aggressive phenotype. PALB2 is a predisposition gene for breast and ovarian cancer, and it is reasonable that in this study, more PALB2 mutation carriers had family histories of breast and/or ovarian cancer than noncarriers. Intriguingly, compared with noncarriers, PALB2 mutation carriers here also more frequently had a family history of cancers other than breast and ovarian cancer, and the underlying mechanism needs to be further investigated.
Treatment regimens and effects were not assessed for PALB2 mutation carriers in this study. A poly(adenosine diphosphate ribose) polymerase (PARP) inhibitor has been successfully used in patients with breast or ovarian cancer carrying BRCA1 or BRCA2 germline mutations. 22 , 23 Although there is still a lack of solid clinical evidence for the use of PARP inhibitors in PALB2‐associated cancer treatment, independent studies have provided evidence that PALB2‐deficient cells are sensitive to PARP inhibitors. 24 , 25 This suggests a potential treatment option for patients with breast cancer and PALB2 germline mutations.
The preeminent strength of this study is that it was a large, population‐based study. All the recruited patients with breast cancer were unselected, and the number of PALB2 mutation carriers was sufficient for analysis. This study comprehensively pictured the spectrum and penetrance of PALB2 germline mutations in the Chinese breast cancer population. This study calculated the breast cancer risk for PALB2 germline mutation carriers and illustrated characteristics of PALB2‐related breast cancer in China. However, there are still several limitations to our study. There was quite a bit of missing data for the tumor size and lymph node status of PALB2 mutation noncarriers, although the proportions of bigger tumors (tumor size ≥ 2 cm, 46%) and positive lymph nodes (39%) were similar to those reported before (results for PALB2 mutation noncarriers in Poland [n = 12,413]: tumor size ≥ 2 cm, 48%; positive lymph nodes, 44% 10 ; our single‐center results published previously [n = 2203]: tumor size > 2 cm, 43%; positive lymph nodes, 38% 12 ). Treatment regimens and effects were not evaluated for PALB2 mutation carriers because the mortality rate of those carriers was too low to support the analysis of treatment effects. In addition, we did not investigate the survival of PALB2 mutation carriers and noncarriers because at the time of this writing the follow‐up for those patients had not been fully finished, and this analysis will be presented in the future.
In conclusion, this study has revealed a comprehensive spectrum of PALB2 germline mutations and characteristics of PALB2‐related breast cancer in China. We showed that 0.97% of unselected BRCA1/2‐negative patients with breast cancer carried deleterious PALB2 germline mutations in this large Chinese cohort. PALB2 germline mutations conferred a moderately increased risk for breast cancer but profoundly increased breast cancer risk for those aged 30 years or younger. Patients with breast cancer and PALB2 germline mutations had distinctive clinical characteristics. An analysis of survival and different treatment effects for PALB2 mutation carriers needs to be further investigated.
Funding Support
This study was supported by the Key Program of the Natural Science Foundation of Zhejiang Province (grant LZ16H160002), the Zhejiang Provincial Program for the Cultivation of High‐Level Innovative Health Talents, the Preclinical and Multicenter Basket Clinical Trial of the Multi‐Kinase Inhibitor TT‐00420 (2019ZX09301158), the National Natural Science Foundation of China (grant 81702866), and the Fundamental Research Funds for the Central Universities (grant 2019FZJD009).
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Jiaojiao Zhou: Data analysis, writing of the manuscript, recruitment of the patients, collection of the blood samples and clinical information, and approval of the final manuscript. Honglian Wang: Data analysis, writing of the manuscript, gene sequencing, and approval of the final manuscript. Fangmeng Fu: Data analysis and approval of the final manuscript. Zhanwen Li: Recruitment of the patients, collection of the blood samples and clinical information, and approval of the final manuscript. Qingjian Feng: Recruitment of the patients, collection of the blood samples and clinical information, and approval of the final manuscript. Weizhu Wu: Recruitment of the patients, collection of the blood samples and clinical information, and approval of the final manuscript. Yun Liu: Planning and design of the study, recruitment of the patients, collection of the blood samples and clinical information, gene sequencing, and approval of the final manuscript. Chuan Wang: Data analysis, recruitment of the patients, collection of the blood samples and clinical information, and approval of the final manuscript. Yiding Chen: Planning and design of the study, data analysis, recruitment of the patients, collection of the blood samples and clinical information, and approval of the final manuscript.
Supporting information
Table S1
Table S2
Table S3
The first 3 authors contributed equally to this article.
We gratefully thank Professor Shu Zheng and Ting Chen (Zhejiang University) for their great support of our study. We are particularly grateful to Dr. Yingying Mao (Zhejiang Chinese Medical University) for reviewing the statistical analysis of this study and to Dr. Chenchen Zhu (Stanford University School of Medicine) for drawing the figures in this article.
Co-corresponding authors: Chuan Wang, MD, PhD, Department of Breast Surgery, Affiliated Union Hospital, Fujian Medical University, 29 Xinquan Rd, Fuzhou, Fujian, China 350001 (chuanwang1968@yahoo.com); Yun Liu, PhD, Key Laboratory of Molecular Medicine, Ministry of Education, Department of Biochemistery and Molecular Biology, Shanghai Medical College, Fudan University, Shanghai, China 200032 (superliuyun@fudan.edu.cn).
Contributor Information
Yun Liu, Email: superliuyun@fudan.edu.cn.
Chuan Wang, Email: chuanwang1968@yahoo.com.
Yiding Chen, Email: ydchen@zju.edu.cn.
References
- 1. Zhang F, Ma J, Wu J, et al. PALB2 links BRCA1 and BRCA2 in the DNA‐damage response. Curr Biol. 2009;19:524‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xia B, Sheng Q, Nakanishi K, et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22:719‐729. [DOI] [PubMed] [Google Scholar]
- 3. Sy SMH, Huen MSY, Zhu Y, Chen J. PALB2 regulates recombinational repair through chromatin association and oligomerization. J Biol Chem. 2009;284:18302‐18310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rahman N, Seal S, Thompson D, et al. PALB2, which encodes a BRCA2‐interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2006;39:165‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erkko H, Xia B, Nikkila J, et al. A recurrent mutation in PALB2 in Finnish cancer families. Nature. 2007;446:316‐319. [DOI] [PubMed] [Google Scholar]
- 6. Antoniou AC, Casadei S, Heikkinen T, et al. Breast‐cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2‐interacting protein PALB2 to familial breast cancer. Cancer Res. 2011;71:2222‐2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janatova M, Kleibl Z, Stribrna J, et al. The PALB2 gene is a strong candidate for clinical testing in BRCA1‐ and BRCA2‐negative hereditary breast cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:2323‐2332. [DOI] [PubMed] [Google Scholar]
- 9. Papi L, Putignano AL, Congregati C, et al. A PALB2 germline mutation associated with hereditary breast cancer in Italy. Fam Cancer. 2010;9:181‐185. [DOI] [PubMed] [Google Scholar]
- 10. Cybulski C, Kluzniak W, Huzarski T, et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. Lancet Oncol. 2015;16:638‐644. [DOI] [PubMed] [Google Scholar]
- 11. Zhang K, Zhou J, Zhu X, et al. Germline mutations of PALB2 gene in a sequential series of Chinese patients with breast cancer. Breast Cancer Res Treat. 2017;166:865‐873. [DOI] [PubMed] [Google Scholar]
- 12. Deng M, Chen HH, Zhu X, et al. Prevalence and clinical outcomes of germline mutations in BRCA1/2 and PALB2 genes in 2769 unselected breast cancer patients in China. Int J Cancer. 2019;145:1517‐1528. [DOI] [PubMed] [Google Scholar]
- 13. Ducy M, Sesma‐Sanz L, Guitton‐Sert L, et al. The tumor suppressor PALB2: inside out. Trends Biochem Sci. 2019;44:226‐240. [DOI] [PubMed] [Google Scholar]
- 14. Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34:1460‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun J, Meng H, Yao L, et al. Germline mutations in cancer susceptibility genes in a large series of unselected breast cancer patients. Clin Cancer Res. 2017;23:6113‐6119. [DOI] [PubMed] [Google Scholar]
- 17. Cao AY, Huang J, Hu Z, et al. The prevalence of PALB2 germline mutations in BRCA1/BRCA2 negative Chinese women with early onset breast cancer or affected relatives. Breast Cancer Res Treat. 2008;114:457‐462. [DOI] [PubMed] [Google Scholar]
- 18. Yang XR, Devi BCR, Sung H, et al. Prevalence and spectrum of germline rare variants in BRCA1/2 and PALB2 among breast cancer cases in Sarawak, Malaysia. Breast Cancer Res Treat. 2017;165:687‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catucci I, Peterlongo P, Ciceri S, et al. PALB2 sequencing in Italian familial breast cancer cases reveals a high‐risk mutation recurrent in the province of Bergamo. Genet Med. 2014;16:688‐694. [DOI] [PubMed] [Google Scholar]
- 20. Tedaldi G, Tebaldi M, Zampiga V, et al. Multiple‐gene panel analysis in a case series of 255 women with hereditary breast and ovarian cancer. Oncotarget. 2017;8:47064‐47075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heikkinen T, Karkkainen H, Aaltonen K, et al. The breast cancer susceptibility mutation PALB2 1592delT is associated with an aggressive tumor phenotype. Clin Cancer Res. 2009;15:3214‐3222. [DOI] [PubMed] [Google Scholar]
- 22. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523‐533. [DOI] [PubMed] [Google Scholar]
- 23. Weil MK, Chen AP. PARP inhibitor treatment in ovarian and breast cancer. Curr Probl Cancer. 2011;35:7‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buisson R, Dion‐Cote AM, Coulombe Y, et al. Cooperation of breast cancer proteins PALB2 and piccolo BRCA2 in stimulating homologous recombination. Nat Struct Mol Biol. 2010;17:1247‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith MA, Hampton OA, Reynolds CP, et al. Initial testing (stage 1) of the PARP inhibitor BMN 673 by the pediatric preclinical testing program: PALB2 mutation predicts exceptional in vivo response to BMN 673. Pediatr Blood Cancer. 2015;62:91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3


